Role for diet in normal gut barrier function: developing guidance within the framework of food-labeling regulations
- PMID: 31125257
- PMCID: PMC6689735
- DOI: 10.1152/ajpgi.00063.2019
Role for diet in normal gut barrier function: developing guidance within the framework of food-labeling regulations
Abstract
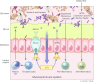
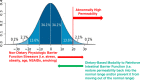
A reduction in intestinal barrier function is currently believed to play an important role in pathogenesis of many diseases, as it facilitates passage of injurious factors such as lipopolysaccharide, peptidoglycan, whole bacteria, and other toxins to traverse the barrier to damage the intestine or enter the portal circulation. Currently available evidence in animal models and in vitro systems has shown that certain dietary interventions can be used to reinforce the intestinal barrier to prevent the development of disease. The relevance of these studies to human health is unknown. Herein, we define the components of the intestinal barrier, review available modalities to assess its structure and function in humans, and review the available evidence in model systems or perturbations in humans that diet can be used to fortify intestinal barrier function. Acknowledging the technical challenges and the present gaps in knowledge, we provide a conceptual framework by which evidence could be developed to support the notion that diet can reinforce human intestinal barrier function to restore normal function and potentially reduce the risk for disease. Such evidence would provide information on the development of healthier diets and serve to provide a framework by which federal agencies such as the US Food and Drug Administration can evaluate evidence linking diet with normal human structure/function claims focused on reducing risk of disease in the general public.
Keywords: diet; function; gut barrier structure; permeability.
Conflict of interest statement
B. J. Lyle provides technical advice and scientific writing consultancy to a range of clients in the private for profit as well as non-profit food and research sector. She is a paid nutrition advisor to the ILSI NA committee that sponsored this project. M. Camilleri: relevant disclosures related to IBS-diarrhea: NIH funding R01-DK115950 and research grants from Novartis (research studies on CLN452) and Allergan (research studies on elobixibat). G. D. Wu: Relevant disclosures related to the gut microbiome and diet include research funding from Seres Therapeutics, Intercept Pharmaceuticals, and Takeda; scientific advisory boards include Danone and Biocodex; consultant agreement with Hitachi. J. Sonnenburg: Cofounder of Novome Biotechnologies, Inc., January, Inc.; scientific advisory board of Clorox/Renew Life, Kaleido Biotechnologies, Second Genome, Gnubiotics Sciences; research funding from Second Genome, Clorox/Renew Life, Abbott Laboratories. Neither of the other authors has any conflicts of interest, financial or otherwise, to report.
Figures

References
-
- Araki Y, Fujiyama Y, Andoh A, Nakamura F, Shimada M, Takaya H, Bamba T. Hydrophilic and hydrophobic bile acids exhibit different cytotoxicities through cytolysis, interleukin-8 synthesis and apoptosis in the intestinal epithelial cell lines. IEC-6 and Caco-2 cells. Scand J Gastroenterol 36: 533–539, 2001. doi:10.1080/003655201750153430. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

