The effects of trans-chalcone and chalcone 4 hydrate on the growth of Babesia and Theileria
- PMID: 31125333
- PMCID: PMC6534319
- DOI: 10.1371/journal.pntd.0007030
The effects of trans-chalcone and chalcone 4 hydrate on the growth of Babesia and Theileria
Abstract
Background: Chemotherapy is a principle tool for the control and prevention of piroplasmosis. The search for a new chemotherapy against Babesia and Theileria parasites has become increasingly urgent due to the toxic side effects of and developed resistance to the current drugs. Chalcones have attracted much attention due to their diverse biological activities. With the aim to discover new drugs and drug targets, in vitro and in vivo antibabesial activity of trans-chalcone (TC) and chalcone 4 hydrate (CH) alone and combined with diminazene aceturate (DA), clofazimine (CF) and atovaquone (AQ) were investigated.
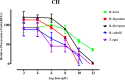
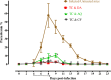
Methodology/principal findings: The fluorescence-based assay was used for evaluating the inhibitory effect of TC and CH on four Babesia species, including B. bovis, B. bigemina, B. divergens, B. caballi, and T. equi, the combination with DA, CF, and AQ on in vitro cultures, and on the multiplication of a B. microti-infected mouse model. The cytotoxicity of compounds was tested on Madin-Darby bovine kidney (MDBK), mouse embryonic fibroblast (NIH/3T3), and human foreskin fibroblast (HFF) cell lines. The half maximal inhibitory concentration (IC50) values of TC and CH against B. bovis, B. bigemina, B. divergens, B. caballi, and T. equi were 69.6 ± 2.3, 33.3 ± 1.2, 64.8 ± 2.5, 18.9 ± 1.7, and 14.3 ± 1.6 μM and 138.4 ± 4.4, 60.9 ± 1.1, 82.3 ± 2.3, 27.9 ± 1.2, and 19.2 ± 1.5 μM, respectively. In toxicity assays, TC and CH affected the viability of MDBK, NIH/3T3, and HFF cell lines the with half maximum effective concentration (EC50) values of 293.9 ± 2.9, 434.4 ± 2.7, and 498 ± 3.1 μM and 252.7 ± 1.7, 406.3 ± 9.7, and 466 ± 5.7 μM, respectively. In the mouse experiment, TC reduced the peak parasitemia of B. microti by 71.8% when administered intraperitoneally at 25 mg/kg. Combination therapies of TC-DA and TC-CF were more potent against B. microti infection in mice than their monotherapies.
Conclusions/significance: In conclusion, both TC and CH inhibited the growth of Babesia and Theileria in vitro, and TC inhibited the growth of B. microti in vivo. Therefore, TC and CH could be candidates for the treatment of piroplasmosis after further studies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Bock R, Jackson L, Vos ADE, Jorgensen W (2004) Babesiosis of cattle. Parasitology 129: S247–269 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

