Aicardi-Goutières syndrome gene Rnaseh2c is a metastasis susceptibility gene in breast cancer
- PMID: 31125342
- PMCID: PMC6553800
- DOI: 10.1371/journal.pgen.1008020
Aicardi-Goutières syndrome gene Rnaseh2c is a metastasis susceptibility gene in breast cancer
Abstract
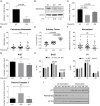
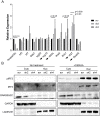
Breast cancer is the second leading cause of cancer-related deaths in the United States, with the majority of these deaths due to metastatic lesions rather than the primary tumor. Thus, a better understanding of the etiology of metastatic disease is crucial for improving survival. Using a haplotype mapping strategy in mouse and shRNA-mediated gene knockdown, we identified Rnaseh2c, a scaffolding protein of the heterotrimeric RNase H2 endoribonuclease complex, as a novel metastasis susceptibility factor. We found that the role of Rnaseh2c in metastatic disease is independent of RNase H2 enzymatic activity, and immunophenotyping and RNA-sequencing analysis revealed engagement of the T cell-mediated adaptive immune response. Furthermore, the cGAS-Sting pathway was not activated in the metastatic cancer cells used in this study, suggesting that the mechanism of immune response in breast cancer is different from the mechanism proposed for Aicardi-Goutières Syndrome, a rare interferonopathy caused by RNase H2 mutation. These results suggest an important novel, non-enzymatic role for RNASEH2C during breast cancer progression and add Rnaseh2c to a panel of genes we have identified that together could determine patients with high risk for metastasis. These results also highlight a potential new target for combination with immunotherapies and may contribute to a better understanding of the etiology of Aicardi-Goutières Syndrome autoimmunity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
RNase H2 catalytic core Aicardi-Goutières syndrome-related mutant invokes cGAS-STING innate immune-sensing pathway in mice.J Exp Med. 2016 Mar 7;213(3):329-36. doi: 10.1084/jem.20151464. Epub 2016 Feb 15. J Exp Med. 2016. PMID: 26880576 Free PMC article.
-
Phenotypic variation in Aicardi-Goutières syndrome explained by cell-specific IFN-stimulated gene response and cytokine release.J Immunol. 2015 Apr 15;194(8):3623-33. doi: 10.4049/jimmunol.1401334. Epub 2015 Mar 13. J Immunol. 2015. PMID: 25769924
-
Ribonuclease H2 mutations induce a cGAS/STING-dependent innate immune response.EMBO J. 2016 Apr 15;35(8):831-44. doi: 10.15252/embj.201593339. Epub 2016 Feb 22. EMBO J. 2016. PMID: 26903602 Free PMC article.
-
Aicardi-Goutières syndrome: clues from the RNase H2 knock-out mouse.J Mol Med (Berl). 2013 Nov;91(11):1235-40. doi: 10.1007/s00109-013-1061-x. Epub 2013 Jun 7. J Mol Med (Berl). 2013. PMID: 23744109 Review.
-
Dirty Fish Versus Squeaky Clean Mice: Dissecting Interspecies Differences Between Animal Models of Interferonopathy.Front Immunol. 2021 Jan 15;11:623650. doi: 10.3389/fimmu.2020.623650. eCollection 2020. Front Immunol. 2021. PMID: 33519829 Free PMC article. Review.
Cited by
-
Emerging Concepts in Vector Development for Glial Gene Therapy: Implications for Leukodystrophies.Front Cell Neurosci. 2021 Jun 22;15:661857. doi: 10.3389/fncel.2021.661857. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34239416 Free PMC article. Review.
-
SMARCD1 is an essential expression-restricted metastasis modifier.Commun Biol. 2024 Oct 10;7(1):1299. doi: 10.1038/s42003-024-07018-3. Commun Biol. 2024. PMID: 39390150 Free PMC article.
-
INFIMA leverages multi-omics model organism data to identify effector genes of human GWAS variants.Genome Biol. 2021 Aug 23;22(1):241. doi: 10.1186/s13059-021-02450-8. Genome Biol. 2021. PMID: 34425882 Free PMC article.
-
Distinct features of ribonucleotides within genomic DNA in Aicardi-Goutières syndrome (AGS)-ortholog mutants of Saccharomyces cerevisiae.bioRxiv [Preprint]. 2023 Oct 2:2023.10.02.560505. doi: 10.1101/2023.10.02.560505. bioRxiv. 2023. Update in: iScience. 2024 May 16;27(6):110012. doi: 10.1016/j.isci.2024.110012. PMID: 37873120 Free PMC article. Updated. Preprint.
-
Significance of Aneuploidy in Predicting Prognosis and Treatment Response of Uveal Melanoma.Curr Med Chem. 2025;32(3):579-594. doi: 10.2174/0109298673286788240123044411. Curr Med Chem. 2025. PMID: 38504568
References
-
- Surveillance, Epidemiology, and End Results (SEER) Program 2016 [cited 2016]. http://seer.cancer.gov/statfacts/html/breast.html.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

