Delivery of different genes into pre- and post-synaptic neocortical interneurons connected by GABAergic synapses
- PMID: 31125364
- PMCID: PMC6534327
- DOI: 10.1371/journal.pone.0217094
Delivery of different genes into pre- and post-synaptic neocortical interneurons connected by GABAergic synapses
Abstract
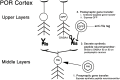
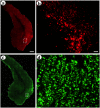
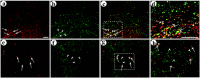
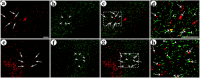
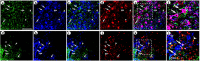
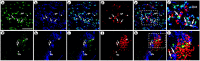
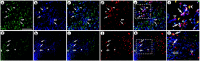
Local neocortical circuits play critical roles in information processing, including synaptic plasticity, circuit physiology, and learning, and GABAergic inhibitory interneurons have key roles in these circuits. Moreover, specific neurological disorders, including schizophrenia and autism, are associated with deficits in GABAergic transmission in these circuits. GABAergic synapses represent a small fraction of neocortical synapses, and are embedded in complex local circuits that contain many neuron and synapse types. Thus, it is challenging to study the physiological roles of GABAergic inhibitory interneurons and their synapses, and to develop treatments for the specific disorders caused by dysfunction at these GABAergic synapses. To these ends, we report a novel technology that can deliver different genes into pre- and post-synaptic neocortical interneurons connected by a GABAergic synapse: First, standard gene transfer into the presynaptic neurons delivers a synthetic peptide neurotransmitter, containing three domains, a dense core vesicle sorting domain, a GABAA receptor-binding domain, a single-chain variable fragment anti-GABAA ß2 or ß3, and the His tag. Second, upon release, this synthetic peptide neurotransmitter binds to GABAA receptors on the postsynaptic neurons. Third, as the synthetic peptide neurotransmitter contains the His tag, antibody-mediated, targeted gene transfer using anti-His tag antibodies is selective for these neurons. We established this technology by expressing the synthetic peptide neurotransmitter in GABAergic neurons in the middle layers of postrhinal cortex, and the delivering the postsynaptic vector into connected GABAergic neurons in the upper neocortical layers. Targeted gene transfer was 61% specific for the connected neurons, but untargeted gene transfer was only 21% specific for these neurons. This technology may support studies on the roles of GABAergic inhibitory interneurons in circuit physiology and learning, and support gene therapy treatments for specific disorders associated with deficits at GABAergic synapses.
Conflict of interest statement
A.I.G. has equity in Alkermes Inc. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



Similar articles
-
Separate Gene Transfers into Pre- and Postsynaptic Neocortical Neurons Connected by mGluR5-Containing Synapses.J Mol Neurosci. 2019 Aug;68(4):549-564. doi: 10.1007/s12031-019-01317-9. Epub 2019 Apr 10. J Mol Neurosci. 2019. PMID: 30972540 Free PMC article.
-
Delivery of different genes into presynaptic and postsynaptic neocortical neurons connected by a BDNF-TrkB synapse.Brain Res. 2019 Jun 1;1712:16-24. doi: 10.1016/j.brainres.2019.01.038. Epub 2019 Jan 30. Brain Res. 2019. PMID: 30710509
-
Targeted gene transfer of different genes to presynaptic and postsynaptic neocortical neurons connected by a glutamatergic synapse.Brain Res. 2012 Sep 14;1473:173-84. doi: 10.1016/j.brainres.2012.07.024. Epub 2012 Jul 20. Brain Res. 2012. PMID: 22820303 Free PMC article.
-
Role of GABAA R trafficking in the plasticity of inhibitory synapses.J Neurochem. 2016 Dec;139(6):997-1018. doi: 10.1111/jnc.13742. Epub 2016 Sep 30. J Neurochem. 2016. PMID: 27424566 Review.
-
GABA(B) and group I metabotropic glutamate receptors in the striatopallidal complex in primates.J Anat. 2000 May;196 ( Pt 4)(Pt 4):555-76. doi: 10.1046/j.1469-7580.2000.19640555.x. J Anat. 2000. PMID: 10923987 Free PMC article. Review.
Cited by
-
Efficient gene transfers into neocortical neurons connected by NMDA NR1-containing synapses.J Neurosci Methods. 2019 Nov 1;327:108390. doi: 10.1016/j.jneumeth.2019.108390. Epub 2019 Aug 9. J Neurosci Methods. 2019. PMID: 31404560 Free PMC article.
-
Separate Gene Transfers into Pre- and Postsynaptic Neocortical Neurons Connected by mGluR5-Containing Synapses.J Mol Neurosci. 2019 Aug;68(4):549-564. doi: 10.1007/s12031-019-01317-9. Epub 2019 Apr 10. J Mol Neurosci. 2019. PMID: 30972540 Free PMC article.
-
Recombinant Antibodies in Basic Neuroscience Research.Curr Protoc Neurosci. 2020 Dec;94(1):e106. doi: 10.1002/cpns.106. Curr Protoc Neurosci. 2020. PMID: 33151027 Free PMC article. Review.
References
-
- Hubel DH, Wiesel TN. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc Royal Soc London—Series B. 1977;198:1–59. - PubMed
-
- Dudai Y. The Neurobiology of Memory. Oxford, England: Oxford Univ. Press; 1989.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

