Longitudinal effects of Nucleos(t)ide analogue therapy in chronic hepatitis B patients and the utility of non-invasive fibrosis markers during treatment: A single-center experience for up to 17 years
- PMID: 31125632
- PMCID: PMC6620142
- DOI: 10.1016/j.antiviral.2019.05.007
Longitudinal effects of Nucleos(t)ide analogue therapy in chronic hepatitis B patients and the utility of non-invasive fibrosis markers during treatment: A single-center experience for up to 17 years
Abstract
Background: Fibrosis regression has been associated with nucleoside analogue (NA) treatment in chronic hepatitis B (CHB) patients. Although non-invasive fibrosis markers have been evaluated in CHB, their utility for monitoring on-treatment histologic regression has not been evaluated.
Aims: To characterize improvements in disease severity and the utility of non-invasive biomarkers in CHB NA treated patients.
Methods: Histology, labs, AST-to-platelet ratio index, and Fibrosis-4 (Fib-4) from treatment-naïve CHB patients were evaluated at baseline and longitudinally. Relative change from baseline to various time points during treatment were evaluated. Correlative analysis of APRI and Fib-4 with histology was performed longitudinally.
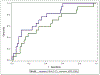
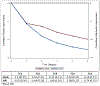
Results: 80 CHB patients (84% male, median age 45 (IQR 32, 54)) with histology up to 17 years (median 6(IQR 3.9, 8.0)) years were studied. Median baseline Ishak fibrosis was 3 (IQR 2, 4), histologic activity index (HAI) inflammation was 9 (IQR 7, 11), and AUROC of fibrosis markers for detecting cirrhosis (Ishak ≥ 5) was >0.64. HAI improved at a rate of 54% during year 1 and 37% in year 2, both greater than in the remaining follow-up periods. Within the first year, fibrosis improved by 35%, greater than all other time periods. Non-invasive biomarkers began to correlate with histology beyond 4 years (APRI: 4-6 years: r = 0.33, p = 0.03; ≥6 years: r = 0.41, p = 0.009; Fib-4: ≥6 years: r = 0.35, p = 0.03).
Conclusion: Early dynamic changes in histology occur in CHB patients on NA followed by linear improvements. Non-invasive fibrosis biomarkers do not capture these dynamic changes and may demonstrate clinical utility beyond 4 years of treatment.
Keywords: Fibrosis; Hepatitis B; Inflammation; Non-invasive markers of fibrosis; Nucleoside analogues.
Published by Elsevier B.V.
Conflict of interest statement
Figures


References
-
- Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–25. - PubMed
-
- Trépo C, Chan HLY, Lok A. Hepatitis B virus infection. The Lancet 2014;384:2053–2063. - PubMed
-
- Grossi G, Viganò M, Loglio A, et al. Hepatitis B virus long-term impact of antiviral therapy nucleot(s)ide analogues (NUCs). Liver international : official journal of the International Association for the Study of the Liver 2017;37 Suppl 1:45–51. - PubMed
-
- Younossi ZM, Stepanova M, Younossi I, et al. Long-Term Effects of Treatment for Chronic HBV Infection on Patient-Reported Outcomes. Clin Gastroenterol Hepatol 2018. - PubMed
-
- Chen CJ, Yang HI. Natural history of chronic hepatitis B REVEALed. J Gastroenterol Hepatol 2011;26:628–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

