Large-scale changes in cortical dynamics triggered by repetitive somatosensory electrical stimulation
- PMID: 31126339
- PMCID: PMC6534962
- DOI: 10.1186/s12984-019-0520-1
Large-scale changes in cortical dynamics triggered by repetitive somatosensory electrical stimulation
Abstract
Background: Repetitive somatosensory electrical stimulation (SES) of forelimb peripheral nerves is a promising therapy; studies have shown that SES can improve motor function in stroke subjects with chronic deficits. However, little is known about how SES can directly modulate neural dynamics. Past studies using SES have primarily used noninvasive methods in human subjects. Here we used electrophysiological recordings from the rodent primary motor cortex (M1) to assess how SES affects neural dynamics at the level of single neurons as well as at the level of mesoscale dynamics.
Methods: We performed acute extracellular recordings in 7 intact adult Long Evans rats under ketamine-xylazine anesthesia while they received transcutaneous SES. We recorded single unit spiking and local field potentials (LFP) in the M1 contralateral to the stimulated arm. We then compared neural firing rate, spike-field coherence (SFC), and power spectral density (PSD) before and after stimulation.
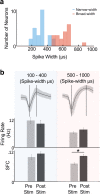
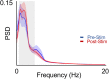
Results: Following SES, the firing rate of a majority of neurons changed significantly from their respective baseline values. There was, however, a diversity of responses; some neurons increased while others decreased their firing rates. Interestingly, SFC, a measure of how a neuron's firing is coupled to mesoscale oscillatory dynamics, increased specifically in the δ-band, also known as the low frequency band (0.3- 4 Hz). This increase appeared to be driven by a change in the phase-locking of broad-spiking, putative pyramidal neurons. These changes in the low frequency range occurred without a significant change in the overall PSD.
Conclusions: Repetitive SES significantly and persistently altered the local cortical dynamics of M1 neurons, changing both firing rates as well as the SFC magnitude in the δ-band. Thus, SES altered the neural firing and coupling to ongoing mesoscale dynamics. Our study provides evidence that SES can directly modulate cortical dynamics.
Keywords: Low frequency oscillations; Motor cortex; Peripheral nerve; Somatosensory electrical stimulation (SES); Spiking dynamics.
Conflict of interest statement
KG has submitted a provisional patent application for closed-loop SES. The results presented in this manuscript are not a part of the provisional patent application. AH, MB and TG do not have any competing interests.
Figures




Similar articles
-
Epidural cerebellar stimulation drives widespread neural synchrony in the intact and stroke perilesional cortex.J Neuroeng Rehabil. 2021 May 26;18(1):89. doi: 10.1186/s12984-021-00881-9. J Neuroeng Rehabil. 2021. PMID: 34039346 Free PMC article.
-
Differential Effects of Open- and Closed-Loop Intracortical Microstimulation on Firing Patterns of Neurons in Distant Cortical Areas.Cereb Cortex. 2020 May 14;30(5):2879-2896. doi: 10.1093/cercor/bhz281. Cereb Cortex. 2020. PMID: 31832642 Free PMC article.
-
Dissociation between sustained single-neuron spiking and transient β-LFP oscillations in primate motor cortex.J Neurophysiol. 2017 Apr 1;117(4):1524-1543. doi: 10.1152/jn.00651.2016. Epub 2017 Jan 18. J Neurophysiol. 2017. PMID: 28100654 Free PMC article.
-
Direct and crossed effects of somatosensory stimulation on neuronal excitability and motor performance in humans.Neurosci Biobehav Rev. 2014 Nov;47:22-35. doi: 10.1016/j.neubiorev.2014.07.013. Epub 2014 Jul 23. Neurosci Biobehav Rev. 2014. PMID: 25064816 Review.
-
Neural dynamics and information representation in microcircuits of motor cortex.Front Neural Circuits. 2013 May 3;7:85. doi: 10.3389/fncir.2013.00085. eCollection 2013. Front Neural Circuits. 2013. PMID: 23653596 Free PMC article. Review.
Cited by
-
Movement-dependent electrical stimulation for volitional strengthening of cortical connections in behaving monkeys.Proc Natl Acad Sci U S A. 2022 Jul 5;119(27):e2116321119. doi: 10.1073/pnas.2116321119. Epub 2022 Jun 27. Proc Natl Acad Sci U S A. 2022. PMID: 35759657 Free PMC article.
-
Dynamic electrophysiological changes in abnormal brain cavities post-ischemic stroke.Front Neurosci. 2025 May 16;19:1565255. doi: 10.3389/fnins.2025.1565255. eCollection 2025. Front Neurosci. 2025. PMID: 40454248 Free PMC article.
-
Non-invasive electrical stimulation of peripheral nerves for the management of tremor.J Neurol Sci. 2022 Apr 15;435:120195. doi: 10.1016/j.jns.2022.120195. Epub 2022 Feb 19. J Neurol Sci. 2022. PMID: 35220113 Free PMC article. Review.
-
Cortical processing during robot and functional electrical stimulation.Front Syst Neurosci. 2023 Mar 21;17:1045396. doi: 10.3389/fnsys.2023.1045396. eCollection 2023. Front Syst Neurosci. 2023. PMID: 37025164 Free PMC article.
-
Epidural cerebellar stimulation drives widespread neural synchrony in the intact and stroke perilesional cortex.J Neuroeng Rehabil. 2021 May 26;18(1):89. doi: 10.1186/s12984-021-00881-9. J Neuroeng Rehabil. 2021. PMID: 34039346 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

