Dopamine Depletion Affects Vocal Acoustics and Disrupts Sensorimotor Adaptation in Songbirds
- PMID: 31126913
- PMCID: PMC6565373
- DOI: 10.1523/ENEURO.0190-19.2019
Dopamine Depletion Affects Vocal Acoustics and Disrupts Sensorimotor Adaptation in Songbirds
Abstract
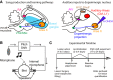
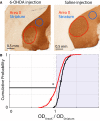
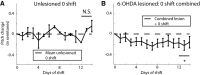
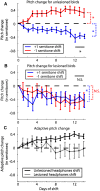
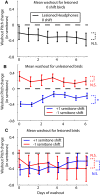
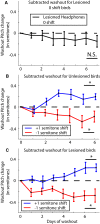
Dopamine is hypothesized to convey error information in reinforcement learning tasks with explicit appetitive or aversive cues. However, during motor skill learning feedback signals arise from an animal's evaluation of sensory feedback resulting from its own behavior, rather than any external reward or punishment. It has previously been shown that intact dopaminergic signaling from the ventral tegmental area/substantia nigra pars compacta (VTA/SNc) complex is necessary for vocal learning when songbirds modify their vocalizations to avoid hearing distorted auditory feedback (playbacks of white noise). However, it remains unclear whether dopaminergic signaling underlies vocal learning in response to more naturalistic errors (pitch-shifted feedback delivered via headphones). We used male Bengalese finches (Lonchura striata var. domestica) to test the hypothesis that the necessity of dopamine signaling is shared between the two types of learning. We combined 6-hydroxydopamine (6-OHDA) lesions of dopaminergic terminals within Area X, a basal ganglia nucleus critical for song learning, with a headphones learning paradigm that shifted the pitch of auditory feedback and compared their learning to that of unlesioned controls. We found that 6-OHDA lesions affected song behavior in two ways. First, over a period of days lesioned birds systematically lowered their pitch regardless of the presence or absence of auditory errors. Second, 6-OHDA lesioned birds also displayed severe deficits in sensorimotor learning in response to pitch-shifted feedback. Our results suggest roles for dopamine in both motor production and auditory error processing, and a shared mechanism underlying vocal learning in response to both distorted and pitch-shifted auditory feedback.
Keywords: Bengalese finch; basal ganglia; dopamine; sensorimotor adaptation; songbird; vocal learning.
Copyright © 2019 Saravanan et al.
Figures
Similar articles
-
Shared mechanisms of auditory and non-auditory vocal learning in the songbird brain.Elife. 2022 Sep 15;11:e75691. doi: 10.7554/eLife.75691. Elife. 2022. PMID: 36107757 Free PMC article.
-
Dopaminergic Contributions to Vocal Learning.J Neurosci. 2016 Feb 17;36(7):2176-89. doi: 10.1523/JNEUROSCI.3883-15.2016. J Neurosci. 2016. PMID: 26888928 Free PMC article.
-
Vocal generalization depends on gesture identity and sequence.J Neurosci. 2014 Apr 16;34(16):5564-74. doi: 10.1523/JNEUROSCI.5169-13.2014. J Neurosci. 2014. PMID: 24741046 Free PMC article.
-
Variations on a theme: Songbirds, variability, and sensorimotor error correction.Neuroscience. 2015 Jun 18;296:48-54. doi: 10.1016/j.neuroscience.2014.09.068. Epub 2014 Oct 14. Neuroscience. 2015. PMID: 25305664 Free PMC article. Review.
-
Songbird: a unique animal model for studying the molecular basis of disorders of vocal development and communication.Exp Anim. 2015;64(3):221-30. doi: 10.1538/expanim.15-0008. Epub 2015 Apr 24. Exp Anim. 2015. PMID: 25912323 Free PMC article. Review.
Cited by
-
Actor-critic reinforcement learning in the songbird.Curr Opin Neurobiol. 2020 Dec;65:1-9. doi: 10.1016/j.conb.2020.08.005. Epub 2020 Sep 6. Curr Opin Neurobiol. 2020. PMID: 32898752 Free PMC article. Review.
-
Mapping the vocal circuitry of Alston's singing mouse with pseudorabies virus.J Comp Neurol. 2022 Aug;530(12):2075-2099. doi: 10.1002/cne.25321. Epub 2022 Apr 6. J Comp Neurol. 2022. PMID: 35385140 Free PMC article.
-
Neural mechanism of dopamine modulating singing related behavior in songbirds: an updated review.PeerJ. 2025 Jun 5;13:e19500. doi: 10.7717/peerj.19500. eCollection 2025. PeerJ. 2025. PMID: 40487051 Free PMC article. Review.
-
Unilateral vocal nerve resection alters neurogenesis in the avian song system in a region-specific manner.PLoS One. 2021 Aug 31;16(8):e0256709. doi: 10.1371/journal.pone.0256709. eCollection 2021. PLoS One. 2021. PMID: 34464400 Free PMC article.
-
Shared mechanisms of auditory and non-auditory vocal learning in the songbird brain.Elife. 2022 Sep 15;11:e75691. doi: 10.7554/eLife.75691. Elife. 2022. PMID: 36107757 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
