CSD-Induced Arterial Dilatation and Plasma Protein Extravasation Are Unaffected by Fremanezumab: Implications for CGRP's Role in Migraine with Aura
- PMID: 31127003
- PMCID: PMC6650995
- DOI: 10.1523/JNEUROSCI.0232-19.2019
CSD-Induced Arterial Dilatation and Plasma Protein Extravasation Are Unaffected by Fremanezumab: Implications for CGRP's Role in Migraine with Aura
Abstract
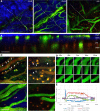
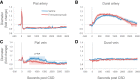
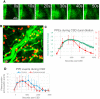
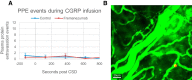
Cortical spreading depression (CSD) is a wave of neuronal depolarization thought to underlie migraine aura. Calcitonin gene-related peptide (CGRP) is a potent vasodilator involved in migraine pathophysiology. Evidence for functional connectivity between CSD and CGRP has triggered scientific interest in the possibility that CGRP antagonism may disrupt vascular responses to CSD and the ensuing plasma protein extravasation (PPE). Using imaging tools that allow us to generate continuous, live, high-resolution views of spatial and temporal changes that affect arteries and veins in the dura and pia, we determined the extent to which CGRP contributes to the induction of arterial dilatation or PPE by CSD in female rats, and how these events are affected by the anti-CGRP monoclonal antibody (anti-CGRP-mAb) fremanezumab. We found that the CSD-induced brief dilatation and prolonged constriction of pial arteries, prolonged dilatation of dural arteries and PPE are all unaffected by fremanezumab, whereas the brief constriction and prolonged dilatation of pial veins are affected. In comparison, although CGRP infusion gave rise to the expected dilatation of dural arteries, which was effectively blocked by fremanezumab, it did not induce dilatation in pial arteries, pial veins, or dural veins. It also failed to induce PPE. Regardless of whether the nociceptors become active before or after the induction of arterial dilatation or PPE by CSD, the inability of fremanezumab to prevent them suggests that these events are not mediated by CGRP, a conclusion with important implications for our understanding of the mechanism of action of anti-CGRP-mAbs in migraine prevention.SIGNIFICANCE STATEMENT The current study identifies fundamental differences between two commonly used models of migraine, CSD induction and systemic CGRP infusion. It raises the possibility that conclusions drawn from one model may not be true or relevant to the other. It sharpens the need to accept the view that there is more than one truth to migraine pathophysiology and that it is unlikely that one theory will explain all types of migraine headache or the mechanisms of action of drugs that prevent it. Regarding the latter, it is concluded that not all vascular responses in the meninges are born alike and, consequently, that drugs that prevent vascular dilatation through different molecular pathways may have different therapeutic outcomes in different types of migraine.
Keywords: CGRP; CSD; PPE; aura; migraine; vasodilation.
Copyright © 2019 the authors.
Figures




Similar articles
-
Selective Inhibition of Trigeminovascular Neurons by Fremanezumab: A Humanized Monoclonal Anti-CGRP Antibody.J Neurosci. 2017 Jul 26;37(30):7149-7163. doi: 10.1523/JNEUROSCI.0576-17.2017. Epub 2017 Jun 22. J Neurosci. 2017. PMID: 28642283 Free PMC article.
-
Fremanezumab-A Humanized Monoclonal Anti-CGRP Antibody-Inhibits Thinly Myelinated (Aδ) But Not Unmyelinated (C) Meningeal Nociceptors.J Neurosci. 2017 Nov 1;37(44):10587-10596. doi: 10.1523/JNEUROSCI.2211-17.2017. Epub 2017 Sep 29. J Neurosci. 2017. PMID: 28972120 Free PMC article.
-
Involvement of calcitonin gene-related peptide (CGRP) and nitric oxide (NO) in the pial artery dilatation elicited by cortical spreading depression.Brain Res. 1994 Feb 21;637(1-2):204-10. doi: 10.1016/0006-8993(94)91234-3. Brain Res. 1994. PMID: 8180797
-
Cortical spreading depression and calcitonin gene-related peptide: a brief review of current progress.Neuropeptides. 2013 Dec;47(6):463-6. doi: 10.1016/j.npep.2013.10.006. Epub 2013 Oct 23. Neuropeptides. 2013. PMID: 24220568 Review.
-
[Cortical spreading depression (CSD): a neurophysiological correlate of migraine aura].Schmerz. 2008 Oct;22(5):544-6, 548-50. doi: 10.1007/s00482-008-0653-9. Schmerz. 2008. PMID: 18483750 Review. German.
Cited by
-
Migraine Pathophysiology Revisited: Proposal of a New Molecular Theory of Migraine Pathophysiology and Headache Diagnostic Criteria.Int J Mol Sci. 2022 Oct 27;23(21):13002. doi: 10.3390/ijms232113002. Int J Mol Sci. 2022. PMID: 36361791 Free PMC article. Review.
-
Parenchymal neuroinflammatory signaling and dural neurogenic inflammation in migraine.J Headache Pain. 2021 Nov 18;22(1):138. doi: 10.1186/s10194-021-01353-0. J Headache Pain. 2021. PMID: 34794382 Free PMC article. Review.
-
Primary headache disorders: From pathophysiology to neurostimulation therapies.Heliyon. 2023 Mar 23;9(4):e14786. doi: 10.1016/j.heliyon.2023.e14786. eCollection 2023 Apr. Heliyon. 2023. PMID: 37077680 Free PMC article. Review.
-
Efficacy of anti-calcitonin gene-related peptide monoclonal antibodies in hemiplegic migraine: a case report and review of literature.Front Neurol. 2025 Apr 8;16:1579203. doi: 10.3389/fneur.2025.1579203. eCollection 2025. Front Neurol. 2025. PMID: 40264646 Free PMC article.
-
Sex difference in TRPM3 channel functioning in nociceptive and vascular systems: an emerging target for migraine therapy in females?J Headache Pain. 2025 Feb 24;26(1):40. doi: 10.1186/s10194-025-01966-9. J Headache Pain. 2025. PMID: 39994546 Free PMC article. Review.
References
-
- Bigal ME, Edvinsson L, Rapoport AM, Lipton RB, Spierings EL, Diener HC, Burstein R, Loupe PS, Ma Y, Yang R, Silberstein SD (2015) Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 14:1091–1100. 10.1016/S1474-4422(15)00245-8 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
