Evidence for the Interaction of A3 Adenosine Receptor Agonists at the Drug-Binding Site(s) of Human P-glycoprotein (ABCB1)
- PMID: 31127007
- PMCID: PMC6608608
- DOI: 10.1124/mol.118.115295
Evidence for the Interaction of A3 Adenosine Receptor Agonists at the Drug-Binding Site(s) of Human P-glycoprotein (ABCB1)
Abstract
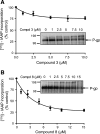
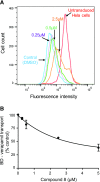
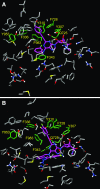
P-glycoprotein (P-gp) is a multidrug transporter that is expressed on the luminal surface of epithelial cells in the kidney, intestine, bile-canalicular membrane in the liver, blood-brain barrier, and adrenal gland. This transporter uses energy of ATP hydrolysis to efflux from cells a variety of structurally dissimilar hydrophobic and amphipathic compounds, including anticancer drugs. In this regard, understanding the interaction with P-gp of drug entities in development is important and highly recommended in current US Food and Drug Administration guidelines. Here we tested the P-gp interaction of some A3 adenosine receptor agonists that are being developed for the treatment of chronic diseases, including rheumatoid arthritis, psoriasis, chronic pain, and hepatocellular carcinoma. Biochemical assays of the ATPase activity of P-gp and by photolabeling P-gp with its transport substrate [125I]-iodoarylazidoprazosin led to the identification of rigidified (N)-methanocarba nucleosides (i.e., compound 3 as a stimulator and compound 8 as a partial inhibitor of P-gp ATPase activity). Compound 8 significantly inhibited boron-dipyrromethene (BODIPY)-verapamil transport mediated by human P-gp (IC50 2.4 ± 0.6 µM); however, the BODIPY-conjugated derivative of 8 (compound 24) was not transported by P-gp. In silico docking of compounds 3 and 8 was performed using the recently solved atomic structure of paclitaxel (Taxol)-bound human P-gp. Molecular modeling studies revealed that both compounds 3 and 8 bind in the same region of the drug-binding pocket as Taxol. Thus, this study indicates that nucleoside derivatives can exhibit varied modulatory effects on P-gp activity, depending on structural functionalization. SIGNIFICANCE STATEMENT: Certain A3 adenosine receptor agonists are being developed for the treatment of chronic diseases. The goal of this study was to test the interaction of these agonists with the human multidrug resistance-linked transporter P-glycoprotein (P-gp). ATPase and photolabeling assays demonstrated that compounds with rigidified (N)-methanocarba nucleosides inhibit the activity of P-gp; however, a fluorescent derivative of one of the compounds was not transported by P-gp. Furthermore, molecular docking studies revealed that the binding site for these compounds overlaps with the site for paclitaxel in the drug-binding pocket. These results suggest that nucleoside derivatives, depending on structural functionalization, can modulate the function of P-gp.
U.S. Government work not protected by U.S. copyright.
Figures



Similar articles
-
Synthesis and Characterization of Bodipy-FL-Cyclosporine A as a Substrate for Multidrug Resistance-Linked P-Glycoprotein (ABCB1).Drug Metab Dispos. 2019 Oct;47(10):1013-1023. doi: 10.1124/dmd.119.087734. Epub 2019 Aug 1. Drug Metab Dispos. 2019. PMID: 31371421 Free PMC article.
-
Drug-protein hydrogen bonds govern the inhibition of the ATP hydrolysis of the multidrug transporter P-glycoprotein.Biochem Pharmacol. 2016 Feb 1;101:40-53. doi: 10.1016/j.bcp.2015.12.007. Epub 2015 Dec 11. Biochem Pharmacol. 2016. PMID: 26686578 Free PMC article.
-
Evidence for the critical role of transmembrane helices 1 and 7 in substrate transport by human P-glycoprotein (ABCB1).PLoS One. 2018 Sep 28;13(9):e0204693. doi: 10.1371/journal.pone.0204693. eCollection 2018. PLoS One. 2018. PMID: 30265721 Free PMC article.
-
Molecular basis of the polyspecificity of P-glycoprotein (ABCB1): recent biochemical and structural studies.Adv Cancer Res. 2015;125:71-96. doi: 10.1016/bs.acr.2014.10.003. Epub 2015 Jan 8. Adv Cancer Res. 2015. PMID: 25640267 Free PMC article. Review.
-
Identification of A3 adenosine receptor agonists as novel non-narcotic analgesics.Br J Pharmacol. 2016 Apr;173(8):1253-67. doi: 10.1111/bph.13446. Epub 2016 Mar 6. Br J Pharmacol. 2016. PMID: 26804983 Free PMC article. Review.
Cited by
-
Adenosine A1R/A3R agonist AST-004 reduces brain infarction in mouse and rat models of acute ischemic stroke.Front Stroke. 2022;1:1010928. doi: 10.3389/fstro.2022.1010928. Epub 2022 Nov 22. Front Stroke. 2022. PMID: 38348128 Free PMC article.
-
Interaction of A3 adenosine receptor ligands with the human multidrug transporter ABCG2.Eur J Med Chem. 2022 Mar 5;231:114103. doi: 10.1016/j.ejmech.2022.114103. Epub 2022 Jan 10. Eur J Med Chem. 2022. PMID: 35152062 Free PMC article.
-
Ligand's Partition to the Lipid Bilayer Should Be Accounted for When Estimating Their Affinity to Proteins.Molecules. 2023 Mar 31;28(7):3136. doi: 10.3390/molecules28073136. Molecules. 2023. PMID: 37049898 Free PMC article.
-
TM2, a novel semi-synthetic taxoid, exerts anti-MDR activity in NSCLC by inhibiting P-gp function and stabilizing microtubule polymerization.Apoptosis. 2022 Dec;27(11-12):1015-1030. doi: 10.1007/s10495-022-01767-4. Epub 2022 Sep 15. Apoptosis. 2022. PMID: 36107354
-
Expanding the repertoire of methanocarba nucleosides from purinergic signaling to diverse targets.RSC Med Chem. 2021 Jul 13;12(11):1808-1825. doi: 10.1039/d1md00167a. eCollection 2021 Nov 17. RSC Med Chem. 2021. PMID: 34825182 Free PMC article. Review.
References
-
- Ambudkar SV. (1998) Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells. Methods Enzymol 292:504–514. - PubMed
-
- Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. (1999) Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol 39:361–398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

