Stress-induced tunneling nanotubes support treatment adaptation in prostate cancer
- PMID: 31127190
- PMCID: PMC6534589
- DOI: 10.1038/s41598-019-44346-5
Stress-induced tunneling nanotubes support treatment adaptation in prostate cancer
Abstract
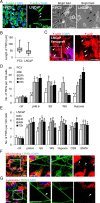
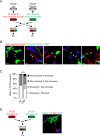
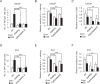
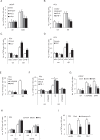
Tunneling nanotubes (TNTs) are actin-based membranous structures bridging distant cells for intercellular communication. We define roles for TNTs in stress adaptation and treatment resistance in prostate cancer (PCa). Androgen receptor (AR) blockade and metabolic stress induce TNTs, but not in normal prostatic epithelial or osteoblast cells. Co-culture assays reveal enhanced TNT formation between stressed and unstressed PCa cells as well as from stressed PCa to osteoblasts. Stress-induced chaperones clusterin and YB-1 localize within TNTs, are transported bi-directionally via TNTs and facilitate TNT formation in PI3K/AKT and Eps8-dependent manner. AR variants, induced by AR antagonism to mediate resistance to AR pathway inhibition, also enhance TNT production and rescue loss of clusterin- or YB-1-repressed TNT formation. TNT disruption sensitizes PCa to treatment-induced cell death. These data define a mechanistic network involving stress induction of chaperone and AR variants, PI3K/AKT signaling, actin remodeling and TNT-mediated intercellular communication that confer stress adaptative cell survival.
Conflict of interest statement
By the way of disclosure of conflict of interest, the University of British Columbia has submitted patent applications on OGX-011, listing Dr. Gleave as inventor.
Figures

References
-
- Gleave M, et al. Progression to androgen independence is delayed by adjuvant treatment with antisense Bcl-2 oligodeoxynucleotides after castration in the LNCaP prostate tumor model. Clin Cancer Res. 1999;5:2891–2898. - PubMed
-
- Miyake H, Nelson C, Rennie PS, Gleave ME. Acquisition of chemoresistant phenotype by overexpression of the antiapoptotic gene testosterone-repressed prostate message-2 in prostate cancer xenograft models. Cancer Res. 2000;60:2547–2554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

