LncRNA H19 initiates microglial pyroptosis and neuronal death in retinal ischemia/reperfusion injury
- PMID: 31127201
- PMCID: PMC7206022
- DOI: 10.1038/s41418-019-0351-4
LncRNA H19 initiates microglial pyroptosis and neuronal death in retinal ischemia/reperfusion injury
Erratum in
-
Correction to: LncRNA H19 initiates microglial pyroptosis and neuronal death in retinal ischemia/reperfusion injury.Cell Death Differ. 2023 Mar;30(3):859. doi: 10.1038/s41418-022-01105-w. Cell Death Differ. 2023. PMID: 36513830 Free PMC article. No abstract available.
Abstract
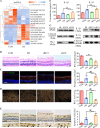
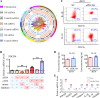
Ischemia-reperfusion (I/R) is a common pathology when the blood supply to an organ was disrupted and then restored. During the reperfusion process, inflammation and tissue injury were triggered, which were mediated by immunocytes and cytokines. However, the mechanisms initiating I/R-induced inflammation and driving immunocytes activation remained largely unknown. In this study, we identified long non-coding RNA (lncRNA)-H19 as the key onset of I/R-induced inflammation. We found that I/R increased lncRNA-H19 expression to significantly promote NLRP3/6 inflammasome imbalance and resulted in microglial pyroptosis, cytokines overproduction, and neuronal death. These damages were effectively inhibited by lncRNA-H19 knockout. Specifically, lncRNA-H19 functioned via sponging miR-21 to facilitate PDCD4 expression and formed a competing endogenous RNA network (ceRNET) in ischemic cascade. LncRNA H19/miR-21/PDCD4 ceRNET can directly regulate I/R-induced sterile inflammation and neuronal lesion in vivo. We thus propose that lncRNA-H19 is a previously unknown danger signals in the molecular and immunological pathways of I/R injury, and pharmacological approaches to inhibit H19 seem likely to become treatment modalities for patients in the near future based on these mechanistic findings.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604. - PubMed
-
- Yang Y, Salayandia VM, Thompson JF, Yang LY, Estrada EY, Yang Y. Attenuation of acute stroke injury in rat brain by minocycline promotes blood-brain barrier remodeling and alternative microglia/macrophage activation during recovery. J Neuroinflamm. 2015;12:26. doi: 10.1186/s12974-015-0245-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

