Follicle-Stimulating Hormone Glycobiology
- PMID: 31127275
- PMCID: PMC6534497
- DOI: 10.1210/en.2019-00001
Follicle-Stimulating Hormone Glycobiology
Abstract
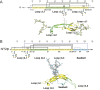
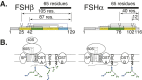
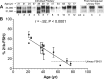
FSH glycosylation varies in two functionally important aspects: microheterogeneity, resulting from oligosaccharide structure variation, and macroheterogeneity, arising from partial FSHβ subunit glycosylation. Although advances in mass spectrometry permit extensive characterization of FSH glycan populations, microheterogeneity remains difficult to illustrate, and comparisons between different studies are challenging because no standard format exists for rendering oligosaccharide structures. FSH microheterogeneity is illustrated using a consistent glycan diagram format to illustrate the large array of structures associated with one hormone. This is extended to commercially available recombinant FSH preparations, which exhibit greatly reduced microheterogeneity at three of four glycosylation sites. Macroheterogeneity is demonstrated by electrophoretic mobility shifts due to the absence of FSHβ glycans that can be assessed by Western blotting of immunopurified FSH. Initially, macroheterogeneity was hoped to matter more than microheterogeneity. However, it now appears that both forms of carbohydrate heterogeneity have to be taken into consideration. FSH glycosylation can reduce its apparent affinity for its cognate receptor by delaying initial interaction with the receptor and limiting access to all of the available binding sites. This is followed by impaired cellular signaling responses that may be related to reduced receptor occupancy or biased signaling. To resolve these alternatives, well-characterized FSH glycoform preparations are necessary.
Copyright © 2019 Endocrine Society.
Figures




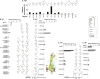

References
-
- Hunzicker-Dunn M, Mayo KE. Gonadotropin signaling in the ovary. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction. Vol 1. 4th ed. Amsterdam, Netherlands: Elsevier; 2015:895–946.
-
- Lee LB, Walker WH. Hormone signaling in the testis. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction. Vol 1. 4th ed. Amsterdam, Netherlands: Elsevier; 2015:637–690.
-
- Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15(2):201–204. - PubMed
-
- Zheng J, Mao J, Cui M, Liu Z, Wang X, Xiong S, Nie M, Wu X. Novel FSHβ mutation in a male patient with isolated FSH deficiency and infertility. Eur J Med Genet. 2017;60(6):335–339. - PubMed
-
- Huhtaniemi I. A short evolutionary history of FSH-stimulated spermatogenesis. Hormones (Athens). 2015;14(4):468–478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

