Fatty acid conjugation enhances potency of antisense oligonucleotides in muscle
- PMID: 31127296
- PMCID: PMC6614804
- DOI: 10.1093/nar/gkz354
Fatty acid conjugation enhances potency of antisense oligonucleotides in muscle
Abstract
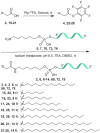
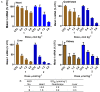
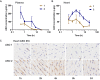
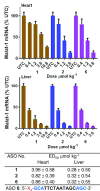
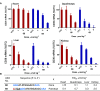
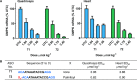
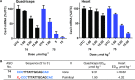
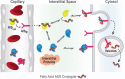
Enhancing the functional uptake of antisense oligonucleotide (ASO) in the muscle will be beneficial for developing ASO therapeutics targeting genes expressed in the muscle. We hypothesized that improving albumin binding will facilitate traversal of ASO from the blood compartment to the interstitium of the muscle tissues to enhance ASO functional uptake. We synthesized structurally diverse saturated and unsaturated fatty acid conjugated ASOs with a range of hydrophobicity. The binding affinity of ASO fatty acid conjugates to plasma proteins improved with fatty acid chain length and highest binding affinity was observed with ASO conjugates containing fatty acid chain length from 16 to 22 carbons. The degree of unsaturation or conformation of double bond appears to have no influence on protein binding or activity of ASO fatty acid conjugates. Activity of fatty acid ASO conjugates correlated with the affinity to albumin and the tightest albumin binder exhibited the highest activity improvement in muscle. Palmitic acid conjugation increases ASO plasma Cmax and improved delivery of ASO to interstitial space of mouse muscle. Conjugation of palmitic acid improved potency of DMPK, Cav3, CD36 and Malat-1 ASOs (3- to 7-fold) in mouse muscle. Our approach provides a foundation for developing more effective therapeutic ASOs for muscle disorders.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures






Similar articles
-
Palmitic acid conjugation enhances potency of tricyclo-DNA splice switching oligonucleotides.Nucleic Acids Res. 2022 Jan 11;50(1):17-34. doi: 10.1093/nar/gkab1199. Nucleic Acids Res. 2022. PMID: 34893881 Free PMC article.
-
Mechanisms of palmitic acid-conjugated antisense oligonucleotide distribution in mice.Nucleic Acids Res. 2020 May 7;48(8):4382-4395. doi: 10.1093/nar/gkaa164. Nucleic Acids Res. 2020. PMID: 32182359 Free PMC article.
-
Propensities of Fatty Acid-Modified ASOs: Self-Assembly vs Albumin Binding.Bioconjug Chem. 2023 May 17;34(5):866-879. doi: 10.1021/acs.bioconjchem.3c00085. Epub 2023 May 5. Bioconjug Chem. 2023. PMID: 37145959
-
An overview of sugar-modified oligonucleotides for antisense therapeutics.Chem Biodivers. 2011 Sep;8(9):1616-41. doi: 10.1002/cbdv.201100081. Chem Biodivers. 2011. PMID: 21922654 Review.
-
Toxicology of antisense therapeutics.Toxicol Appl Pharmacol. 2004 Nov 15;201(1):66-83. doi: 10.1016/j.taap.2004.04.017. Toxicol Appl Pharmacol. 2004. PMID: 15519609 Review.
Cited by
-
Peptide-Oligonucleotide Conjugation: Chemistry and Therapeutic Applications.Curr Issues Mol Biol. 2024 Sep 30;46(10):11031-11047. doi: 10.3390/cimb46100655. Curr Issues Mol Biol. 2024. PMID: 39451535 Free PMC article. Review.
-
Performance of nanoparticles for biomedical applications: The in vitro/in vivo discrepancy.Biophys Rev (Melville). 2022 Feb 1;3(1):011303. doi: 10.1063/5.0073494. eCollection 2022 Mar. Biophys Rev (Melville). 2022. PMID: 38505225 Free PMC article. Review.
-
Antisense technology: A review.J Biol Chem. 2021 Jan-Jun;296:100416. doi: 10.1016/j.jbc.2021.100416. Epub 2021 Feb 16. J Biol Chem. 2021. PMID: 33600796 Free PMC article. Review.
-
Recent Progress and Challenges in the Development of Antisense Therapies for Myotonic Dystrophy Type 1.Int J Mol Sci. 2022 Nov 1;23(21):13359. doi: 10.3390/ijms232113359. Int J Mol Sci. 2022. PMID: 36362145 Free PMC article. Review.
-
RNA-Based Strategies for Cancer Therapy: In Silico Design and Evaluation of ASOs for Targeted Exon Skipping.Int J Mol Sci. 2023 Oct 3;24(19):14862. doi: 10.3390/ijms241914862. Int J Mol Sci. 2023. PMID: 37834310 Free PMC article.
References
-
- Crooke S.T., Witztum J.L., Bennett C.F., Baker B.F.. RNA-targeted therapeutics. Cell Metab. 2018; 27:714–739. - PubMed
-
- Prakash T.P., Graham M.J., Yu J., Carty R., Low A., Chappell A., Schmidt K., Zhao C., Aghajan M., Murray H.F. et al. .. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 2014; 42:8796–8807. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

