Imaging and Spectral Characteristics of Amyloid Plaque Autofluorescence in Brain Slices from the APP/PS1 Mouse Model of Alzheimer's Disease
- PMID: 31127445
- PMCID: PMC6864001
- DOI: 10.1007/s12264-019-00393-6
Imaging and Spectral Characteristics of Amyloid Plaque Autofluorescence in Brain Slices from the APP/PS1 Mouse Model of Alzheimer's Disease
Abstract
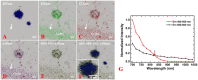
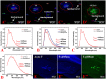
Amyloid deposits are one of the hallmark pathological lesions of Alzheimer's disease (AD). They can be visualized by thioflavin-S, silver impregnation, Congo red staining, and immunohistochemical reactions. However, that amyloid deposits generate blue autofluorescence (auto-F) has been ignored. Here, we report that visible light-induced auto-F of senile plaques (SPs) was detected and validated with conventional methods. Brain slices from APP/PS1 (amyloid precursor protein/presenilin 1) transgenic mice were mounted on slides, rinsed, coverslipped and observed for details of the imaging and spectral characteristics of the auto-F of SPs. Then the slices were treated with the above classic methods for comparative validation. We found that the SP auto-F was greatest under blue-violet excitation with a specific emission spectrum, and was much easier, more sensitive, and reliable than the classic methods. Because it does not damage slices, observation of auto-F can be combined with all post-staining techniques in slices and for brain-wide imaging in AD.
Keywords: Alzheimer’s disease; Amyloid deposits; Autofluorescence; Glial activation; Senile plaques; Spectral imaging.
Conflict of interest statement
The authors claim that there are no conflicts of interest.
Figures






References
-
- Mavrogiorgou P, Gertz HJ, Ferszt R, Wolf R, Bar KJ, Juckell G. Are routine methods good enough to stain senile plaques and neurofibrillary tangles in different brain regions of demented patients? Psychiatr Danub. 2011;23:334–339. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

