A Comprehensive Analysis of Ontogeny of Renal Drug Transporters: mRNA Analyses, Quantitative Proteomics, and Localization
- PMID: 31127606
- PMCID: PMC6777991
- DOI: 10.1002/cpt.1516
A Comprehensive Analysis of Ontogeny of Renal Drug Transporters: mRNA Analyses, Quantitative Proteomics, and Localization
Abstract
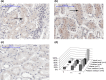
Human renal membrane transporters play key roles in the disposition of renally cleared drugs and endogenous substrates, but their ontogeny is largely unknown. Using 184 human postmortem frozen renal cortical tissues (preterm newborns to adults) and a subset of 62 tissue samples, we measured the mRNA levels of 11 renal transporters and the transcription factor pregnane X receptor (PXR) with quantitative real-time polymerase chain reaction, and protein abundance of nine transporters using liquid chromatography tandem mass spectrometry selective reaction monitoring, respectively. Expression levels of p-glycoprotein, urate transporter 1, organic anion transporter 1, organic anion transporter 3, and organic cation transporter 2 increased with age. Protein levels of multidrug and toxin extrusion transporter 2-K and breast cancer resistance protein showed no difference from newborns to adults, despite age-related changes in mRNA expression. Multidrug and toxin extrusion transporter 1, glucose transporter 2, multidrug resistance-associated protein 2, multidrug resistance-associated protein 4 (MRP4), and PXR expression levels were stable. Using immunohistochemistry, we found that MRP4 localization in pediatric samples was similar to that in adult samples. Collectively, our study revealed that renal drug transporters exhibited different rates and patterns of maturation, suggesting that renal handling of substrates may change with age.
© 2019 The Authors Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work. As an Associate Editor for
Figures



References
-
- Morrissey, K.M. , Stocker, S.L. , Wittwer, M.B. , Xu, L. & Giacomini, K.M. Renal transporters in drug development. Annu. Rev. Pharmacol. Toxicol. 53, 503–529 (2013). - PubMed
-
- Motohashi, H. et al Precise comparison of protein localization among OCT, OAT, and MATE in human kidney. J. Pharm. Sci. 102, 3302–3308 (2013). - PubMed
-
- US Food and Drug Administration . In vitro metabolism‐ and transporter‐mediated drug‐drug interaction studies < https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidan...>. Accessed September 24, 2018.
-
- International Council for Harmonisation . Guidance for Industry. E11 Clinical investigation of medicinal products in the pediatric population. (2000). - PubMed
-
- European Medicines Agency . Guideline on the investigation of drug interactions. Committee for Human Medicinal Products <http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin...>. Accessed April 3, 2018.

