Galectin-1 inhibition induces cell apoptosis through dual suppression of CXCR4 and Ras pathways in human malignant peripheral nerve sheath tumors
- PMID: 31127849
- PMCID: PMC6827838
- DOI: 10.1093/neuonc/noz093
Galectin-1 inhibition induces cell apoptosis through dual suppression of CXCR4 and Ras pathways in human malignant peripheral nerve sheath tumors
Abstract
Background: The Ras signaling pathway is commonly dysregulated in human malignant peripheral nerve sheath tumors (MPNSTs). It is well known that galectin-1 (Gal-1) is essential to stabilize membrane Ras and thereby induce the activation of Ras. However, the role of Gal-1 in MPNST progression remains unknown. The aim of this study was to examine whether Gal-1 knockdown could have an effect on the Ras signaling pathway.
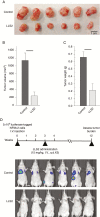
Methods: Cell viability, apoptosis assay, and colony formation were performed to examine the effects of inhibition of Gal-1 in MPNST cells. We used a human MPNST xenograft model to assess growth and metastasis inhibitory effects of Gal-1 inhibitor LLS2.
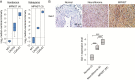
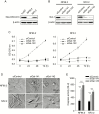
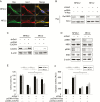
Results: Gal-1 was upregulated in MPNST patients and was highly expressed in MPNST cells. Knockdown of Gal-1 by small interfering (si)RNA in Gal-1 expressing MPNST cells significantly reduces cell proliferation through the suppression of C-X-C chemokine receptor type 4 (CXCR4) and the rat sarcoma viral oncogene homolog (RAS)/extracellular signal-regulated kinase (ERK) pathway, which are important oncogenic signaling in MPNST development. Moreover, Gal-1 knockdown induces apoptosis and inhibits colony formation. LLS2, a novel Gal-1 allosteric small molecule inhibitor, is cytotoxic against MPNST cells and was able to induce apoptosis and suppress colony formation in MPNST cells. LLS2 treatment and Gal-1 knockdown exhibited similar effects on the suppression of CXCR4 and RAS/ERK pathways. More importantly, inhibition of Gal-1 expression or function by treatment with either siRNA or LLS2 resulted in significant tumor responses in an MPNST xenograft model.
Conclusion: Our results identified an oncogenic role of Gal-1 in MPNST and that its inhibitor, LLS2, is a potential therapeutic agent, applied topically or systemically, against MPNST.
Keywords: CXCR4; LLS2; MPNST; RAS; galectin-1.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


Comment in
-
Understanding a complicated Gal-1.Neuro Oncol. 2019 Nov 4;21(11):1341-1343. doi: 10.1093/neuonc/noz165. Neuro Oncol. 2019. PMID: 31538650 Free PMC article. No abstract available.
Similar articles
-
Functional imaging of RAS pathway targeting in malignant peripheral nerve sheath tumor cells and xenografts.Pediatr Blood Cancer. 2020 Dec;67(12):e28639. doi: 10.1002/pbc.28639. Epub 2020 Sep 25. Pediatr Blood Cancer. 2020. PMID: 32975370
-
R-Ras subfamily proteins elicit distinct physiologic effects and phosphoproteome alterations in neurofibromin-null MPNST cells.Cell Commun Signal. 2021 Sep 16;19(1):95. doi: 10.1186/s12964-021-00773-4. Cell Commun Signal. 2021. PMID: 34530870 Free PMC article.
-
RABL6A Is an Essential Driver of MPNSTs that Negatively Regulates the RB1 Pathway and Sensitizes Tumor Cells to CDK4/6 Inhibitors.Clin Cancer Res. 2020 Jun 15;26(12):2997-3011. doi: 10.1158/1078-0432.CCR-19-2706. Epub 2020 Feb 21. Clin Cancer Res. 2020. PMID: 32086342 Free PMC article.
-
The promise of signal transduction in genetically driven sarcomas of the nerve.Exp Neurol. 2018 Jan;299(Pt B):317-325. doi: 10.1016/j.expneurol.2017.08.014. Epub 2017 Aug 30. Exp Neurol. 2018. PMID: 28859862 Review.
-
Unraveling galectin-1 as a novel therapeutic target for cancer.Cancer Treat Rev. 2014 Mar;40(2):307-19. doi: 10.1016/j.ctrv.2013.07.007. Epub 2013 Aug 1. Cancer Treat Rev. 2014. PMID: 23953240 Review.
Cited by
-
Galectin-1 Ameliorates Influenza A H1N1pdm09 Virus-Induced Acute Lung Injury.Front Microbiol. 2020 Jun 12;11:1293. doi: 10.3389/fmicb.2020.01293. eCollection 2020. Front Microbiol. 2020. PMID: 32595629 Free PMC article.
-
Toward Understanding the Mechanisms of Malignant Peripheral Nerve Sheath Tumor Development.Int J Mol Sci. 2021 Aug 10;22(16):8620. doi: 10.3390/ijms22168620. Int J Mol Sci. 2021. PMID: 34445326 Free PMC article. Review.
-
Establishment and characterization of a recurrent malignant peripheral nerve sheath tumor cell line: RsNF.Hum Cell. 2024 Jan;37(1):345-355. doi: 10.1007/s13577-023-01000-7. Epub 2023 Nov 8. Hum Cell. 2024. PMID: 37938540
-
Inhibition of galectins in cancer: Biological challenges for their clinical application.Front Immunol. 2023 Jan 10;13:1104625. doi: 10.3389/fimmu.2022.1104625. eCollection 2022. Front Immunol. 2023. PMID: 36703969 Free PMC article. Review.
-
Understanding a complicated Gal-1.Neuro Oncol. 2019 Nov 4;21(11):1341-1343. doi: 10.1093/neuonc/noz165. Neuro Oncol. 2019. PMID: 31538650 Free PMC article. No abstract available.
References
-
- Ferner RE. Neurofibromatosis 1. Eur J Hum Genet. 2007;15(2):131–138. - PubMed
-
- Van den Brûle FA, Waltregny D, Castronovo V. Increased expression of galectin-1 in carcinoma-associated stroma predicts poor outcome in prostate carcinoma patients. J. Pathol. 2001;193(1):80–87. https://onlinelibrary.wiley.com/doi/abs/10.1002/1096-9896%282000%299999%... - PubMed
-
- Carlini MJ, Roitman P, Nuñez M, et al. . Clinical relevance of galectin-1 expression in non-small cell lung cancer patients. Lung Cancer. 2014;84(1):73–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

