Interactions of Dichlorodiphenyltrichloroethane (DDT) and Dichlorodiphenyldichloroethylene (DDE) With Skeletal Muscle Ryanodine Receptor Type 1
- PMID: 31127943
- PMCID: PMC6657572
- DOI: 10.1093/toxsci/kfz120
Interactions of Dichlorodiphenyltrichloroethane (DDT) and Dichlorodiphenyldichloroethylene (DDE) With Skeletal Muscle Ryanodine Receptor Type 1
Abstract
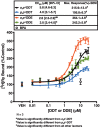
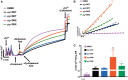
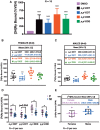
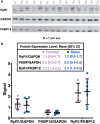
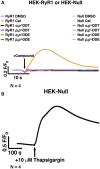
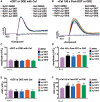
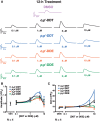
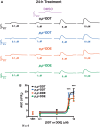
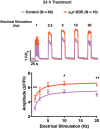
Dichlorodiphenyltrichloroethane (DDT) and its metabolite dichlorodiphenyldichloroethylene (DDE) are ubiquitous in the environment and detected in tissues of living organisms. Although DDT owes its insecticidal activity to impeding closure of voltage-gated sodium channels, it mediates toxicity in mammals by acting as an endocrine disruptor (ED). Numerous studies demonstrate DDT/DDE to be EDs, but studies examining muscle-specific effects mediated by nonhormonal receptors in mammals are lacking. Therefore, we investigated whether o,p'-DDT, p,p'-DDT, o,p'-DDE, and p,p'-DDE (DDx, collectively) alter the function of ryanodine receptor type 1 (RyR1), a protein critical for skeletal muscle excitation-contraction coupling and muscle health. DDx (0.01-10 µM) elicited concentration-dependent increases in [3H]ryanodine ([3H]Ry) binding to RyR1 with o,p'-DDE showing highest potency and efficacy. DDx also showed sex differences in [3H]Ry-binding efficacy toward RyR1, where [3H]Ry-binding in female muscle preparations was greater than male counterparts. Measurements of Ca2+ transport across sarcoplasmic reticulum (SR) membrane vesicles further confirmed DDx can selectively engage with RyR1 to cause Ca2+ efflux from SR stores. DDx also disrupts RyR1-signaling in HEK293T cells stably expressing RyR1 (HEK-RyR1). Pretreatment with DDx (0.1-10 µM) for 100 s, 12 h, or 24 h significantly sensitized Ca2+-efflux triggered by RyR agonist caffeine in a concentration-dependent manner. o,p'-DDE (24 h; 1 µM) significantly increased Ca2+-transient amplitude from electrically stimulated mouse myotubes compared with control and displayed abnormal fatigability. In conclusion, our study demonstrates DDx can directly interact and modulate RyR1 conformation, thereby altering SR Ca2+-dynamics and sensitize RyR1-expressing cells to RyR1 activators, which may ultimately contribute to long-term impairments in muscle health.
Keywords: dichlorodiphenyldichloroethylene; dichlorodiphenyltrichloroethane; ryanodine receptors; skeletal muscle.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
Ryanodine Receptor Type 2: A Molecular Target for Dichlorodiphenyltrichloroethane- and Dichlorodiphenyldichloroethylene-Mediated Cardiotoxicity.Toxicol Sci. 2020 Nov 1;178(1):159-172. doi: 10.1093/toxsci/kfaa139. Toxicol Sci. 2020. PMID: 32894766 Free PMC article.
-
Comparison of Chlorantraniliprole and Flubendiamide Activity Toward Wild-Type and Malignant Hyperthermia-Susceptible Ryanodine Receptors and Heat Stress Intolerance.Toxicol Sci. 2019 Feb 1;167(2):509-523. doi: 10.1093/toxsci/kfy256. Toxicol Sci. 2019. PMID: 30329129 Free PMC article.
-
FKBP12 binding to RyR1 modulates excitation-contraction coupling in mouse skeletal myotubes.J Biol Chem. 2003 Jun 20;278(25):22600-8. doi: 10.1074/jbc.M205866200. Epub 2003 Apr 17. J Biol Chem. 2003. PMID: 12704193
-
Ryanodine receptor isoforms of non-Mammalian skeletal muscle.Front Biosci. 2002 May 1;7:d1184-94. doi: 10.2741/A832. Front Biosci. 2002. PMID: 11991845 Review.
-
Calcium-induced calcium release in skeletal muscle.Physiol Rev. 2009 Oct;89(4):1153-76. doi: 10.1152/physrev.00040.2008. Physiol Rev. 2009. PMID: 19789379 Review.
Cited by
-
Ryanodine Receptor Type 2: A Molecular Target for Dichlorodiphenyltrichloroethane- and Dichlorodiphenyldichloroethylene-Mediated Cardiotoxicity.Toxicol Sci. 2020 Nov 1;178(1):159-172. doi: 10.1093/toxsci/kfaa139. Toxicol Sci. 2020. PMID: 32894766 Free PMC article.
-
Marine and Anthropogenic Bromopyrroles Alter Cellular Ca2+ Dynamics of Murine Cortical Neuronal Networks by Targeting the Ryanodine Receptor and Sarco/Endoplasmic Reticulum Ca2+-ATPase.Environ Sci Technol. 2021 Dec 7;55(23):16023-16033. doi: 10.1021/acs.est.1c05214. Epub 2021 Nov 17. Environ Sci Technol. 2021. PMID: 34788016 Free PMC article.
-
Consensus on the key characteristics of metabolism disruptors.Nat Rev Endocrinol. 2025 Apr;21(4):245-261. doi: 10.1038/s41574-024-01059-8. Epub 2024 Nov 29. Nat Rev Endocrinol. 2025. PMID: 39613954 Review.
-
Type 2 Diabetes Mellitus Mediation by the Disruptive Activity of Environmental Toxicants on Sex Hormone Receptors: In Silico Evaluation.Toxics. 2021 Oct 8;9(10):255. doi: 10.3390/toxics9100255. Toxics. 2021. PMID: 34678951 Free PMC article.
References
-
- Anderson L. J., Liu H., Garcia J. M. (2017). Sex differences in muscle wasting. Adv. Exp. Med. Biol. 1043, 153–197. - PubMed
-
- Aracena P., Tang W., Hamilton S. L., Hidalgo C. (2005). Effects of S-glutathionylation and S-nitrosylation on calmodulin binding to triads and FKBP12 binding to type 1 calcium release channels. Antioxid. Redox Signal. 7, 870–881. - PubMed
-
- Baker M. E., Lathe R. (2018). The promiscuous estrogen receptor: Evolution of physiological estrogens and response to phytochemicals and endocrine disruptors. J. Steroid Biochem. Mol. Biol. 184, 29–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

