N10 -carbonyl-substituted phenothiazines inhibiting lipid peroxidation and associated nitric oxide consumption powerfully protect brain tissue against oxidative stress
- PMID: 31127979
- PMCID: PMC6790564
- DOI: 10.1111/cbdd.13572
N10 -carbonyl-substituted phenothiazines inhibiting lipid peroxidation and associated nitric oxide consumption powerfully protect brain tissue against oxidative stress
Abstract
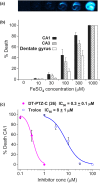
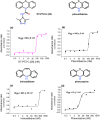
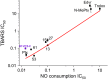
During some investigations into the mechanism of nitric oxide consumption by brain preparations, several potent inhibitors of this process were identified. Subsequent tests revealed the compounds act by inhibiting lipid peroxidation, a trigger for a form of regulated cell death known as ferroptosis. A quantitative structure-activity study together with XED (eXtended Electron Distributions) field analysis allowed a qualitative understanding of the structure-activity relationships. A representative compound N-(3,5-dimethyl-4H-1,2,4-triazol-4-yl)-10H-phenothiazine-10-carboxamide (DT-PTZ-C) was able to inhibit completely oxidative damage brought about by two different procedures in organotypic hippocampal slice cultures, displaying a 30- to 100-fold higher potency than the standard vitamin E analogue, Trolox or edaravone. The compounds are novel, small, drug-like molecules of potential therapeutic use in neurodegenerative disorders and other conditions associated with oxidative stress.
Keywords: XED; antioxidant; ferroptosis; lipid peroxidation; neurodegeneration; nitric oxide; phenothiazine.
© 2019 The Authors. Chemical Biology & Drug Design Published by John Wiley & Sons Ltd.
Figures



Similar articles
-
A subset of N-substituted phenothiazines inhibits NADPH oxidases.Free Radic Biol Med. 2015 Sep;86:239-49. doi: 10.1016/j.freeradbiomed.2015.05.023. Epub 2015 May 23. Free Radic Biol Med. 2015. PMID: 26013584
-
Why Have Clinical Trials of Antioxidants to Prevent Neurodegeneration Failed? - A Cellular Investigation of Novel Phenothiazine-Type Antioxidants Reveals Competing Objectives for Pharmaceutical Neuroprotection.Pharm Res. 2017 Feb;34(2):378-393. doi: 10.1007/s11095-016-2068-0. Epub 2016 Nov 28. Pharm Res. 2017. PMID: 27896592
-
Increased lipid peroxidation, apoptosis and selective cytotoxicity in colon cancer cell line LoVo and its doxorubicin-resistant subline LoVo/Dx in the presence of newly synthesized phenothiazine derivatives.Biomed Pharmacother. 2018 Oct;106:624-636. doi: 10.1016/j.biopha.2018.06.170. Epub 2018 Jul 11. Biomed Pharmacother. 2018. PMID: 29990852
-
Chemical structure of phenothiazines and their biological activity.Pharmacol Rep. 2012;64(1):16-23. doi: 10.1016/s1734-1140(12)70726-0. Pharmacol Rep. 2012. PMID: 22580516 Review.
-
Programmed Cell-Death by Ferroptosis: Antioxidants as Mitigators.Int J Mol Sci. 2019 Oct 8;20(19):4968. doi: 10.3390/ijms20194968. Int J Mol Sci. 2019. PMID: 31597407 Free PMC article. Review.
Cited by
-
Cytokines and Madness: A Unifying Hypothesis of Schizophrenia Involving Interleukin-22.Int J Mol Sci. 2024 Nov 11;25(22):12110. doi: 10.3390/ijms252212110. Int J Mol Sci. 2024. PMID: 39596179 Free PMC article. Review.
-
Novel Insights into Psychosis and Antipsychotic Interventions: From Managing Symptoms to Improving Outcomes.Int J Mol Sci. 2024 May 28;25(11):5904. doi: 10.3390/ijms25115904. Int J Mol Sci. 2024. PMID: 38892092 Free PMC article. Review.
-
Insomnia in Forensic Detainees: Is Salience Network the Common Pathway for Sleep, Neuropsychiatric, and Neurodegenerative Disorders?J Clin Med. 2024 Mar 15;13(6):1691. doi: 10.3390/jcm13061691. J Clin Med. 2024. PMID: 38541916 Free PMC article. Review.
-
Oxytosis/Ferroptosis in Neurodegeneration: the Underlying Role of Master Regulator Glutathione Peroxidase 4 (GPX4).Mol Neurobiol. 2024 Mar;61(3):1507-1526. doi: 10.1007/s12035-023-03646-8. Epub 2023 Sep 19. Mol Neurobiol. 2024. PMID: 37725216 Review.
-
Antiferroptotic Activity of Phenothiazine Analogues: A Novel Therapeutic Strategy for Oxidative Stress Related Disease.ACS Med Chem Lett. 2020 Sep 15;11(11):2165-2173. doi: 10.1021/acsmedchemlett.0c00293. eCollection 2020 Nov 12. ACS Med Chem Lett. 2020. PMID: 33214825 Free PMC article.
References
-
- Abe, K. , Aoki, M. , Tsuji, S. , Itoyama, Y. , Sobue, G. , Togo, M. , … Grp, E. M.‐A. S. (2017). Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: A randomised, double‐blind, placebo‐controlled trial. Lancet Neurology, 16(7), 505–512. 10.1016/S1474-4422(17)30115-1 - DOI - PubMed
-
- Borges, M. B. D. , Dos Santos, C. G. , Yokomizo, C. H. , Sood, R. , Vitovic, P. , Kinnunen, P. K. J. , … Nantes, I. L. (2010). Characterization of hydrophobic interaction and antioxidant properties of the phenothiazine nucleus in mitochondrial and model membranes. Free Radical Research, 44(9), 1054–1063. 10.3109/10715762.2010.498826 - DOI - PubMed
-
- Cacabelos, D. , Ayala, V. , Ramirez‐Nunez, O. , Granado‐Serrano, A. B. , Boada, J. , Serrano, J. C. , … Portero‐Otin, M. (2014). Dietary lipid unsaturation influences survival and oxidative modifications of an amyotrophic lateral sclerosis model in a gender‐specific manner. Neuromolecular Medicine, 16(4), 669–685. 10.1007/s12017-014-8317-7 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

