Prolonged ocular exposure leads to retinal lesions in mice
- PMID: 31128100
- PMCID: PMC7332086
- DOI: 10.1016/j.exer.2019.05.012
Prolonged ocular exposure leads to retinal lesions in mice
Abstract
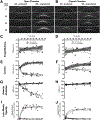
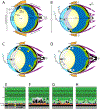
Retinal lesions in the posterior pole of laboratory mice occur due to native, developmental abnormalities or as a consequence of environmental or experimental conditions. In this study, we investigated the rate and extent of retinal lesions as a result of prolonged ocular exposure following general anesthesia. Following experimental preparation induction procedures (EPIP) involving general anesthesia, mydriasis/cycloplegia, and topical anesthesia to the cornea, two ocular recovery conditions (protected and unprotected) were tested within two different animal recovery chambers (open or closed). The anterior and posterior poles were evaluated for the development of retinal lesions using digital color photography, scanning laser ophthalmoscopy, and spectral-domain optical coherence during anesthesia recovery and up to 2.5 months thereafter. In some mice, electroretinograms, histological and immunohistological evaluations were performed to assess functional and structural changes that accompanied the retinal lesions detected by in vivo imaging. Our data suggests that prolonged ocular surface exposure to circulating ambient room air leads to significant anterior and posterior segment ocular complications. The most abundant, semi-reversible complication observed was the development of lesions in the outer retina, which had a 90% probability of occurring after 45 min of exposure. The lesions mostly resolved short-term, but functional and imaging evidence suggest that some perturbations to the outer retina may persist one or more months following initial development.
Keywords: Abnormality; Adrenergic agonist; Choriocapillaris; Imaging; Ischemia-reperfusion injury; Ketamine; Lesion; Mice; Outer segments; Phenylephrine; Photoreceptor; Retina; Retinal pigmented epithelium; Subretinal cyst; Vortex vein; Xylazine.
Copyright © 2019. Published by Elsevier Ltd.
Figures







Similar articles
-
Effects of common anesthetics on eye movement and electroretinogram.Doc Ophthalmol. 2011 Jun;122(3):163-76. doi: 10.1007/s10633-011-9271-4. Epub 2011 Apr 26. Doc Ophthalmol. 2011. PMID: 21519880 Free PMC article.
-
Topical mydriatics affect light-evoked retinal responses in anesthetized mice.Invest Ophthalmol Vis Sci. 2010 Jan;51(1):567-76. doi: 10.1167/iovs.09-4168. Epub 2009 Aug 6. Invest Ophthalmol Vis Sci. 2010. PMID: 19661232 Free PMC article.
-
MULTIMODAL IMAGING IN VORTEX VEIN VARICES.Retin Cases Brief Rep. 2019 Summer;13(3):260-265. doi: 10.1097/ICB.0000000000000575. Retin Cases Brief Rep. 2019. PMID: 28333853
-
[Pathophysiology of macular diseases--morphology and function].Nippon Ganka Gakkai Zasshi. 2011 Mar;115(3):238-74; discussion 275. Nippon Ganka Gakkai Zasshi. 2011. PMID: 21476310 Review. Japanese.
-
Autofluorescence from the outer retina and subretinal space: hypothesis and review.Retina. 2008 Jan;28(1):5-35. doi: 10.1097/IAE.0b013e318158eca4. Retina. 2008. PMID: 18185134 Review.
Cited by
-
Altered Corneal Epithelial Dendritic Cell Morphology and Phenotype Following Acute Exposure to Hyperosmolar Saline.Invest Ophthalmol Vis Sci. 2021 Feb 1;62(2):38. doi: 10.1167/iovs.62.2.38. Invest Ophthalmol Vis Sci. 2021. PMID: 33625479 Free PMC article.
-
Treatment Potential for LCA5-Associated Leber Congenital Amaurosis.Invest Ophthalmol Vis Sci. 2020 May 11;61(5):30. doi: 10.1167/iovs.61.5.30. Invest Ophthalmol Vis Sci. 2020. PMID: 32428231 Free PMC article.
-
Role of monocarboxylate transporters in regulating metabolic homeostasis in the outer retina: Insight gained from cell-specific Bsg deletion.FASEB J. 2020 Apr;34(4):5401-5419. doi: 10.1096/fj.201902961R. Epub 2020 Feb 28. FASEB J. 2020. PMID: 32112484 Free PMC article.
-
Exploring laser-induced acute and chronic retinal vein occlusion mouse models: Development, temporal in vivo imaging, and application perspectives.PLoS One. 2024 Jun 17;19(6):e0305741. doi: 10.1371/journal.pone.0305741. eCollection 2024. PLoS One. 2024. PMID: 38885229 Free PMC article.
-
Glucose uptake by GLUT1 in photoreceptors is essential for outer segment renewal and rod photoreceptor survival.FASEB J. 2022 Aug;36(8):e22428. doi: 10.1096/fj.202200369R. FASEB J. 2022. PMID: 35766190 Free PMC article.
References
-
- Arras M, Autenried P, Rettich A, Spaeni D, Rulicke T, 2001. Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adverse effects, and anesthesia depth. Comp. Med 51 (5), 443–456. - PubMed
-
- Bell BA, Kaul C, Rayborn ME, Hollyfield JG, 2012. Baseline imaging reveals pre-existing retinal abnormalities in mice. Adv. Exp. Med. Biol 723, 459–469. - PubMed
-
- Bermudez MA, Vicente AF, Romero MC, Arcos MD, Abalo JM, Gonzalez F, 2011. Time course of cold cataract development in anesthetized mice. Curr. Eye Res 36 (3), 278–284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

