Hydrogel-based ocular drug delivery systems: Emerging fabrication strategies, applications, and bench-to-bedside manufacturing considerations
- PMID: 31128143
- PMCID: PMC6629478
- DOI: 10.1016/j.jconrel.2019.05.034
Hydrogel-based ocular drug delivery systems: Emerging fabrication strategies, applications, and bench-to-bedside manufacturing considerations
Abstract
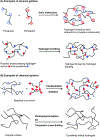
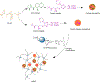
The physiological barriers of the eye pose challenges to the delivery of the array of therapeutics for ocular diseases. Hydrogels have been widely explored for medical applications and introduce possible solutions to overcoming the medication challenges of the ocular environment. While the innovations in drug encapsulation and release mechanisms, biocompatibility, and treatment duration have become highly sophisticated, the challenge of widespread application of hydrogel formulations in the clinic is still apparent. This article reviews the latest hydrogel formulations and their associated chemistries for use in ocular therapies, spanning from external anterior to internal posterior regions of the eye in order to evaluate the state of recent research. This article discusses the utility of hydrogels in soft contact lens, wound dressings, intraocular lens, vitreous substitutes, vitreous drug release hydrogels, and cell-based therapies for regeneration. Additional focus is placed on the pre-formulation, formulation, and manufacturing considerations of the hydrogels based on individual components (polymer chains, linkers, and therapeutics), final hydrogel product, and required preparations for clinical/commercial applications, respectively.
Keywords: Drug delivery; Hydrogel; Injectable; Sterilization; Topical.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures
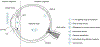




Similar articles
-
Hydrogel-based ocular drug delivery systems for hydrophobic drugs.Eur J Pharm Sci. 2020 Nov 1;154:105503. doi: 10.1016/j.ejps.2020.105503. Epub 2020 Aug 1. Eur J Pharm Sci. 2020. PMID: 32745587 Review.
-
Hydrogels in ophthalmic applications.Eur J Pharm Biopharm. 2015 Sep;95(Pt B):227-38. doi: 10.1016/j.ejpb.2015.05.016. Epub 2015 May 29. Eur J Pharm Biopharm. 2015. PMID: 26032290 Review.
-
Development of ocular drug delivery systems using molecularly imprinted soft contact lenses.Drug Dev Ind Pharm. 2015 May;41(5):703-13. doi: 10.3109/03639045.2014.948451. Epub 2014 Aug 12. Drug Dev Ind Pharm. 2015. PMID: 25113431 Review.
-
Hydrogels-based ophthalmic drug delivery systems for treatment of ocular diseases.Mater Sci Eng C Mater Biol Appl. 2021 Aug;127:112212. doi: 10.1016/j.msec.2021.112212. Epub 2021 May 29. Mater Sci Eng C Mater Biol Appl. 2021. PMID: 34225864 Review.
-
Revolutionizing Ophthalmic Care: A Review of Ocular Hydrogels from Pathologies to Therapeutic Applications.Curr Eye Res. 2025 Jan;50(1):1-17. doi: 10.1080/02713683.2024.2396385. Epub 2024 Sep 11. Curr Eye Res. 2025. PMID: 39261982 Review.
Cited by
-
Preparation and Stability Study of an Injectable Hydrogel for Artificial Intraocular Lenses.Polymers (Basel). 2024 Sep 10;16(18):2562. doi: 10.3390/polym16182562. Polymers (Basel). 2024. PMID: 39339025 Free PMC article.
-
Retinal Diseases: The Next Frontier in Pharmacodelivery.Pharmaceutics. 2022 Apr 21;14(5):904. doi: 10.3390/pharmaceutics14050904. Pharmaceutics. 2022. PMID: 35631490 Free PMC article. Review.
-
Hydrogel Contact Lenses Embedded with Amine-Functionalized Large-Pore Mesoporous Silica Nanoparticles with Extended Hyaluronic Acid Release.Nanomaterials (Basel). 2023 Aug 28;13(17):2441. doi: 10.3390/nano13172441. Nanomaterials (Basel). 2023. PMID: 37686949 Free PMC article.
-
Role of Sterilization on In Situ Gel-Forming Polymer Stability.Polymers (Basel). 2024 Oct 21;16(20):2943. doi: 10.3390/polym16202943. Polymers (Basel). 2024. PMID: 39458771 Free PMC article. Review.
-
Long-Acting Ocular Injectables: Are We Looking In The Right Direction?Adv Sci (Weinh). 2024 Feb;11(8):e2306463. doi: 10.1002/advs.202306463. Epub 2023 Nov 28. Adv Sci (Weinh). 2024. PMID: 38018313 Free PMC article. Review.
References
-
- ABELL RG, DARIAN-SMITH E, KAN JB, ALLEN PL, EWE SYP & VOTE BJ 2015. Femtosecond laser-assisted cataract surgery versus standard phacoemulsificati on cataract surgery: Outcomes and safety in more than 4000 cases at a single center. Journal of Cataract and Refractive Surgery, 41, 47–52. - PubMed
-
- ALEXANDER A, AJAZUDDIN KHAN,J & SARAF S 2014. Polyethylene glycol (PEG)-Poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications. European Journal of Pharmaceutics and Biopharmaceutics, 88, 575–585. - PubMed

