Systems Biology Approach Pinpoints Minimum Requirements for Auxin Distribution during Fruit Opening
- PMID: 31128274
- PMCID: PMC6557309
- DOI: 10.1016/j.molp.2019.05.003
Systems Biology Approach Pinpoints Minimum Requirements for Auxin Distribution during Fruit Opening
Abstract
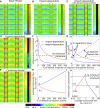
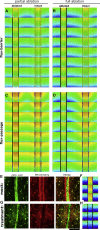
The phytohormone auxin is implied in steering various developmental decisions during plant morphogenesis in a concentration-dependent manner. Auxin maxima have been shown to maintain meristematic activity, for example, of the root apical meristem, and position new sites of outgrowth, such as during lateral root initiation and phyllotaxis. More recently, it has been demonstrated that sites of auxin minima also provide positional information. In the developing Arabidopsis fruit, auxin minima are required for correct differentiation of the valve margin. It remains unclear, however, how this auxin minimum is generated and maintained. Here, we employ a systems biology approach to model auxin transport based on experimental observations. This allows us to determine the minimal requirements for its establishment. Our simulations reveal that two alternative processes-which we coin "flux-barrier" and "flux-passage"-are both able to generate an auxin minimum, but under different parameter settings. Both models are in principle able to yield similar auxin profiles but present qualitatively distinct patterns of auxin flux. The models were tested by tissue-specific inducible ablation, revealing that the auxin minimum in the fruit is most likely generated by a flux-passage process. Model predictions were further supported through 3D PIN localization imaging and implementing experimentally observed transporter localization. Through such an experimental-modeling cycle, we predict how the auxin minimum gradually matures during fruit development to ensure timely fruit opening and seed dispersal.
Keywords: auxin; fruit development; mathematical modeling; polar auxin transport; systems biology of patterning.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
A regulated auxin minimum is required for seed dispersal in Arabidopsis.Nature. 2009 May 28;459(7246):583-6. doi: 10.1038/nature07875. Nature. 2009. PMID: 19478783
-
Role of the Arabidopsis PIN6 auxin transporter in auxin homeostasis and auxin-mediated development.PLoS One. 2013 Jul 29;8(7):e70069. doi: 10.1371/journal.pone.0070069. Print 2013. PLoS One. 2013. PMID: 23922907 Free PMC article.
-
An INDEHISCENT-Controlled Auxin Response Specifies the Separation Layer in Early Arabidopsis Fruit.Mol Plant. 2016 Jun 6;9(6):857-69. doi: 10.1016/j.molp.2016.03.005. Epub 2016 Mar 16. Mol Plant. 2016. PMID: 26995296
-
Deciphering Auxin-Ethylene Crosstalk at a Systems Level.Int J Mol Sci. 2018 Dec 14;19(12):4060. doi: 10.3390/ijms19124060. Int J Mol Sci. 2018. PMID: 30558241 Free PMC article. Review.
-
Auxin at the shoot apical meristem.Cold Spring Harb Perspect Biol. 2010 Apr;2(4):a001487. doi: 10.1101/cshperspect.a001487. Epub 2010 Mar 24. Cold Spring Harb Perspect Biol. 2010. PMID: 20452945 Free PMC article. Review.
Cited by
-
Cellular mechanism of polarized auxin transport on fruit shape determination revealed by time-lapse live imaging.Plant Reprod. 2024 Nov 21;38(1):1. doi: 10.1007/s00497-024-00513-x. Plant Reprod. 2024. PMID: 39570478
-
Two orthogonal differentiation gradients locally coordinate fruit morphogenesis.Nat Commun. 2024 Apr 4;15(1):2912. doi: 10.1038/s41467-024-47325-1. Nat Commun. 2024. PMID: 38575617 Free PMC article.
References
-
- Abley K., Barbier de Reuille P., Strutt D., Bangham A., Prusinkiewicz P., Marée A.F.M., Grieneisen V.A., Coen E. An intracellular partitioning-based framework for tissue cell polarity in plants and animals. Development. 2013;140:2061–2074. - PubMed
- Abley, K., Barbier de Reuille, P., Strutt, D., Bangham, A., Prusinkiewicz, P., Maree, A.F.M., Grieneisen, V.A., Coen, E., 2013. An intracellular partitioning-based framework for tissue cell polarity in plants and animals. Development 140, 2061-2074. - PubMed
-
- Band L.R., Wells D.M., Fozard J.A., Ghetiu T., French A.P., Pound M.P., Wilson M.H., Yu L., Li W., Hijazi H.I. Systems analysis of auxin transport in the Arabidopsis root apex. Plant Cell. 2014;26:862–875. - PMC - PubMed
- Band, L.R., Wells, D.M., Fozard, J.A., Ghetiu, T., French, A.P., Pound, M.P., Wilson, M.H., Yu, L., Li, W., Hijazi, H.I., et al.., 2014. Systems analysis of auxin transport in the Arabidopsis root apex. Plant Cell 26, 862-875. - PMC - PubMed
-
- Bandyopadhyay A., Blakeslee J.J., Lee O.R., Mravec J., Sauer M., Titapiwatanakun B., Makam S.N., Bouchard R., Geisler M., Martinoia E. Interactions of PIN and PGP auxin transport mechanisms. Biochem. Soc. Trans. 2007;35:137–141. - PubMed
- Bandyopadhyay, A., Blakeslee, J.J., Lee, O.R., Mravec, J., Sauer, M., Titapiwatanakun, B., Makam, S.N., Bouchard, R., Geisler, M., Martinoia, E., et al.., 2007. Interactions of PIN and PGP auxin transport mechanisms. Biochem. Soc. Trans. 35, 137-141. - PubMed
-
- Barbier de Reuille P., Bohn-Courseau I., Ljung K., Morin H., Carraro N., Godin C., Traas J. Computer simulations reveal properties of the cell-cell signaling network at the shoot apex in Arabidopsis. Proc. Natl. Acad. Sci. U S A. 2006;103:1627–1632. - PMC - PubMed
- Barbier de Reuille, P., Bohn-Courseau, I., Ljung, K., Morin, H., Carraro, N., Godin, C., Traas, J., 2006. Computer simulations reveal properties of the cell-cell signaling network at the shoot apex in Arabidopsis. Proc. Natl. Acad. Sci. U S A 103, 1627-1632. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BBS/E/J/00000613/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/J/000PR9788/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J004588/1 /BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/P013511/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources

