A rapid bead-based radioligand binding assay for the determination of target-binding fraction and quality control of radiopharmaceuticals
- PMID: 31128476
- PMCID: PMC6599726
- DOI: 10.1016/j.nucmedbio.2019.04.005
A rapid bead-based radioligand binding assay for the determination of target-binding fraction and quality control of radiopharmaceuticals
Abstract
Introduction: Determination of the target-binding fraction (TBF) of radiopharmaceuticals using cell-based assays is prone to inconsistencies arising from several intrinsic and extrinsic factors. Here, we report a cell-free quantitative method of analysis to determine the TBF of radioligands.
Methods: Magnetic beads functionalized with Ni-NTA or streptavidin were incubated with 1 μg of histidine-tagged or biotinylated antigen of choice for 15 min, followed by incubating 1 ng of the radioligand for 30 min. The beads, supernatant and wash fractions were measured for radioactivity on a gamma counter. The TBF was determined by quantifying the percentage of activity associated with the magnetic beads.
Results: The described method works robustly with a variety of radioisotopes and class of molecules used as radioligands. The entire assay can be completed within 2 h.
Conclusion: The described method yields results in a rapid and reliable manner whilst improving and extending the scope of previously described bead-based radioimmunoassays.
Advances in knowledge: Using a bead-based radioligand binding assay overcomes the limitations of traditional cell-based assays. The described method is applicable to antibody as well as non-antibody based radioligands and is independent of the effect of target antigen density on cells, the choice of radioisotope used for synthesis of the radioligand and the temperature at which the assay is performed.
Implications for patient care: The bead-based radioligand binding assay is significantly easier to perform and is ideally suited for adoption by the radiopharmacy as a quality control method of analysis to fulfill the criteria for release of radiopharmaceuticals in the clinic. The use of this assay is likely to ensure a more reliable validation of radiopharmaceutical quality and result in fewer failed doses, which could ultimately translate to an efficient release of radiopharmaceuticals for administration to patients in the clinic.
Keywords: Immunoreactivity; Lindmo assay; Magnetic beads; Target-binding fraction.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
FINANCIAL DISCLOSURE
JSL has received research funding from MabVax Therapeutics Holdings, Inc. There are no other potential conflicts of interest.
Figures

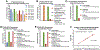

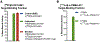
References
-
- Lindmo T, Boven E, Cuttitta F, Fedorko J, and Bunn PA Jr. Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods 1984;72:77–89. - PubMed
-
- Mattes MJ. Limitations of the Lindmo method in determining antibody immunoreactivity. Int J Cancer 1995;61:286–8. - PubMed
-
- Dux R, Kindler-Rohrborn A, Lennartz K, and Rajewsky MF. Determination of immunoreactive fraction and kinetic parameters of a radiolabeled monoclonal antibody in the absence of antigen excess. J Immunol Methods 1991;144:175–83. - PubMed
-
- Rhodes BA, Buckelew JM, Pant KD, and Hinkle GH. Quality control test for immunoreactivity of radiolabeled antibody. Biotechniques 1990;8:70–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

