Pro-apoptotic and anti-angiogenic actions of 2-methoxyestradiol and docosahexaenoic acid, the biologically derived active compounds from flaxseed diet, in preventing ovarian cancer
- PMID: 31128594
- PMCID: PMC6535187
- DOI: 10.1186/s13048-019-0523-3
Pro-apoptotic and anti-angiogenic actions of 2-methoxyestradiol and docosahexaenoic acid, the biologically derived active compounds from flaxseed diet, in preventing ovarian cancer
Abstract
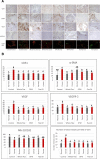
Background: We have previously shown that a whole flaxseed supplemented diet decreased the onset and severity of ovarian cancer in the laying hen, the only known animal model of spontaneous ovarian cancer. Flaxseed is rich in omega-3 fatty acids (OM3FA), mostly α-Linoleic acid (ALA), which gets converted to Docosahexaenoic acid (DHA) by the action of delta-6 desaturase enzyme. Ingestion of flaxseed also causes an increase in production of 2-methoxyestradiol (2MeOE2) via the induction of the CYP1A1 pathway of estrogen metabolism. We have previously reported that the flaxseed diet induces apoptosis via p38-MAPK pathway in chicken tumors. The objective of this study was to investigate the effect of the flaxseed diet on ovarian cancer in chickens, focusing on two hallmarks of cancer, apoptosis and angiogenesis.
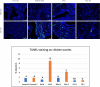
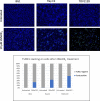
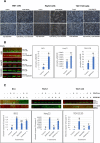
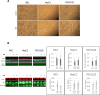
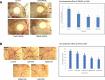
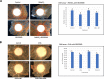
Results: The anti-cancer effects of two active biologically derived compounds of flax diet, 2MeOE2 and DHA, were individually tested on human ovarian cancer cells and in vivo by the Chick Chorioallantoic Membrane (CAM) assay. Our results indicate that a flaxseed-supplemented diet promotes apoptosis and inhibits angiogenesis in chicken tumors but not in normal ovaries. 2MeOE2 promotes apoptosis in human ovarian cancer cells, inhibits angiogenesis on CAM and its actions are dependent on the p38-MAPK pathway. DHA does not have any pro-apoptotic effect on human ovarian cancer cells but has strong anti-angiogenic effects as seen on CAM, but not dependent on the p38-MAPK pathway.
Conclusions: Dietary flaxseed supplementation promotes a pro-apoptotic and anti-angiogenic effect in ovarian tumors, not in normal ovaries. The biologically derived active compounds from flaxseed diet act through different pathways to elicit their respective anti-cancer effects. A flaxseed-supplemented diet is a promising approach for prevention of ovarian cancer as well as having a significant potential as an adjuvant treatment to supplement chemotherapeutic agents for treatment of advanced stages of ovarian cancer.
Keywords: 2-methoxyestradiol; Angiogenesis; Apoptosis; Docosahexaenoic acid; Flaxseed; Ovarian cancer; p38-MAPK.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Whole flaxseed diet alters estrogen metabolism to promote 2-methoxtestradiol-induced apoptosis in hen ovarian cancer.J Nutr Biochem. 2017 Apr;42:117-125. doi: 10.1016/j.jnutbio.2017.01.002. Epub 2017 Jan 23. J Nutr Biochem. 2017. PMID: 28178600 Free PMC article.
-
Flaxseed and its components differentially affect estrogen targets in pre-neoplastic hen ovaries.J Steroid Biochem Mol Biol. 2016 May;159:73-85. doi: 10.1016/j.jsbmb.2016.02.028. Epub 2016 Feb 27. J Steroid Biochem Mol Biol. 2016. PMID: 26925929 Free PMC article.
-
The pro-apoptotic actions of 2-methoxyestradiol against ovarian cancer involve catalytic activation of PKCδ signaling.Oncotarget. 2020 Oct 6;11(40):3646-3659. doi: 10.18632/oncotarget.27760. eCollection 2020 Oct 6. Oncotarget. 2020. PMID: 33088425 Free PMC article.
-
Influence of Flaxseed (Linum usitatissimum) on Female Reproduction.Planta Med. 2023 May;89(6):608-615. doi: 10.1055/a-2013-2966. Epub 2023 Feb 20. Planta Med. 2023. PMID: 36808094 Review.
-
Flaxseed in Diet: A Comprehensive Look at Pros and Cons.Molecules. 2025 Mar 16;30(6):1335. doi: 10.3390/molecules30061335. Molecules. 2025. PMID: 40142110 Free PMC article. Review.
Cited by
-
Anti-Cancer Properties of Flaxseed Proteome.Proteomes. 2023 Nov 16;11(4):37. doi: 10.3390/proteomes11040037. Proteomes. 2023. PMID: 37987317 Free PMC article. Review.
-
Protective Effects of 2-Methoxyestradiol on Acute Isoproterenol-Induced Cardiac Injury in Rats.Saudi Pharm J. 2023 Oct;31(10):101787. doi: 10.1016/j.jsps.2023.101787. Epub 2023 Sep 13. Saudi Pharm J. 2023. PMID: 37766820 Free PMC article.
-
Docosahexaenoic acid (DHA), an omega-3 fatty acid, inhibits tumor growth and metastatic potential of ovarian cancer.Am J Cancer Res. 2020 Dec 1;10(12):4450-4463. eCollection 2020. Am J Cancer Res. 2020. PMID: 33415010 Free PMC article.
-
Analysis of expression and prognosis of KLK7 in ovarian cancer.Open Med (Wars). 2020 Sep 30;15(1):932-939. doi: 10.1515/med-2020-0139. eCollection 2020. Open Med (Wars). 2020. PMID: 33336051 Free PMC article.
-
Oleic Acid, Cholesterol, and Linoleic Acid as Angiogenesis Initiators.ACS Omega. 2020 Aug 10;5(32):20575-20585. doi: 10.1021/acsomega.0c02850. eCollection 2020 Aug 18. ACS Omega. 2020. PMID: 32832811 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

