Extraction of the cardiac waveform from simultaneous multislice fMRI data using slice sorted averaging and a deep learning reconstruction filter
- PMID: 31129302
- PMCID: PMC6592732
- DOI: 10.1016/j.neuroimage.2019.05.049
Extraction of the cardiac waveform from simultaneous multislice fMRI data using slice sorted averaging and a deep learning reconstruction filter
Abstract
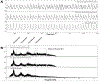
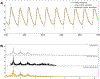
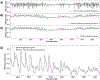
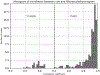
Cardiac signal contamination has long confounded the analysis of blood-oxygenation-level-dependent (BOLD) functional magnetic resonance imaging (fMRI). Cardiac pulsation results in significant BOLD signal changes, especially in and around blood vessels. Until the advent of simultaneous multislice echo-planar imaging (EPI) acquisition, the time resolution of whole brain EPI was insufficient to avoid cardiac aliasing (and acquisitions with repetition times (TRs) under 400-500 ms are still uncommon). As a result, direct detection and removal of the cardiac signal with spectral filters is generally not possible. Modelling methods have been developed to mitigate cardiac contamination, and recently developed techniques permit the visualization of cardiac signal propagation through the brain in undersampled data (e.g., TRs > 1s), which is useful in its own right for finding blood vessels. However, both of these techniques require data from which to estimate cardiac phase, which is generally not available for the data in many large databases of existing imaging data, and even now is not routinely recorded in many fMRI experiments. Here we present a method to estimate the cardiac waveform directly from a multislice fMRI dataset, without additional physiological measurements, such as plethysmograms. The pervasive spatial extent and temporal structure of the cardiac contamination signal across the brain offers an opportunity to exploit the nature of multislice imaging to extract this signal from the fMRI data itself. While any particular slice is recorded at the TR of the imaging experiment, slices are recorded much more quickly - typically from 10 to 20 Hz - sufficiently fast to fully sample the cardiac signal. Using the fairly permissive assumptions that the cardiac signal is a) pseudoperiodic b) somewhat coherent within any given slice, and c) is similarly shaped throughout the brain, we can extract a good estimate of the cardiac phase as a function of time from fMRI data alone. If we make further assumptions about the shape and consistency of cardiac waveforms, we can develop a deep learning filter to greatly improve our estimate of the cardiac waveform.
Keywords: BOLD; Cardiac waveform; Physiological noise; Plethysmogram; fMRI.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures
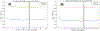



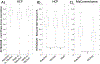


References
-
- Glover GH, Li TQ, and Ress D, Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med, 2000. 44(1): p. 162–7. - PubMed
-
- Birn RM, Diamond JB, Smith MA, and Bandettini PA, Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage, 2006. 31(4): p. 1536–48. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

