Continuous Compressive Force Induces Differentiation of Osteoclasts with High Levels of Inorganic Dissolution
- PMID: 31129676
- PMCID: PMC6556073
- DOI: 10.12659/MSM.913674
Continuous Compressive Force Induces Differentiation of Osteoclasts with High Levels of Inorganic Dissolution
Abstract
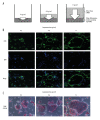
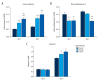
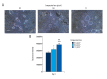
BACKGROUND Osteoclast precursor cells are constitutively differentiated into mature osteoclasts on bone tissues. We previously reported that the continuous stimulation of RAW264.7 precursor cells with compressive force induces the formation of multinucleated giant cells via receptor activator of nuclear factor kappaB (RANK)-RANK ligand (RANKL) signaling. Here, we examined the bone resorptive function of multinucleated osteoclasts induced by continuous compressive force. MATERIAL AND METHODS Cells were continuously stimulated with 0.3, 0.6, and 1.1 g/cm² compressive force created by increasing the amount of the culture solution in the presence of RANKL. Actin ring organization was evaluated by fluorescence microscopy. mRNA expression of genes encoding osteoclastic bone resorption-related enzymes was examined by quantitative real-time reverse transcription-polymerase chain reaction. Mineral resorption was evaluated using calcium phosphate-coated plates. RESULTS Multinucleated osteoclast-like cells with actin rings were observed for all three magnitudes of compressive force, and the area of actin rings increased as a function of the applied force. Carbonic anhydrase II expression as well as calcium elution from the calcium phosphate plate was markedly higher after stimulation with 0.6 and 1.1 g/cm² force than 0.3 g/cm². Matrix metalloproteinase-9 expression decreased and cathepsin K expression increased slightly by the continuous application of compressive force. CONCLUSIONS Our study demonstrated that multinucleated osteoclast-like cells induced by the stimulation of RAW264.7 cells with continuous compressive force exhibit high dissolution of the inorganic phase of bone by upregulating carbonic anhydrase II expression and actin ring formation. These findings improve our understanding of the role of mechanical load in bone remodeling.
Conflict of interest statement
None.
Figures

References
-
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. - PubMed
-
- Pereira M, Petretto E, Gordon S, et al. Common signalling pathways in macrophage and osteoclast multinucleation. J Cell Sci. 2018;131 pii: jcs216267. - PubMed
-
- Väänänen HK, Laitala-Leinonen T. Osteoclast lineage and function. Arch Biochem Biophys. 2008;473:132–38. - PubMed
-
- Skerry TM. The response of bone to mechanical loading and disuse: Fundamental principles and influences on osteoblast/osteocyte homeostasis. Arch Biochem Biophys. 2008;473:117–23. - PubMed
-
- Karlsson MK, Rosengren BE. Training and bone – from health to injury. Scand J Med Sci Sports. 2012;22:e15–23. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

