Safety and efficacy of hydroxytyrosol-based formulation on skin inflammation: in vitro evaluation on reconstructed human epidermis model
- PMID: 31129807
- PMCID: PMC6593001
- DOI: 10.1007/s40199-019-00274-3
Safety and efficacy of hydroxytyrosol-based formulation on skin inflammation: in vitro evaluation on reconstructed human epidermis model
Abstract
Background: Atopic dermatitis is a multifactorial immune-mediated skin disorder characterized by an alteration of epidermal barrier function and onset of skin lesions, which range from mild erythema to severe lichenification. Treatment consists in hydration with possible use of topical or immunomodulatory corticosteroids, which, however sometimes showed side effects. Recently, the interest in natural compounds has grown significantly and among these, hydroxytyrosol (HT) plays a pivotal role due to its strong and well-known anti-inflammatory activity.
Objectives: The aim of this study was to investigate the safety and efficacy of Fenolia® Eudermal Cream 15 (HT-based formulation) on epidermal barrier impaired as consequence of skin injury.
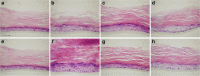
Methods: Whit this purpose, morphologic and structural as well as anti-inflammatory evaluations, after treatment with pro-inflammatory mediators (PBS 1 X and LPS) and HT-based formulation on reconstructed human epidermis (RHE) were carried out by qualitative (hematoxylin/eosin- and immunostaining) and quantitative (MTT assay, IL-1α and IL-8 release by ELISA) techniques. Furthermore, HT absorption through the epidermal barrier was evaluated by RP-LC-DAD analysis.
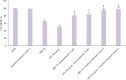
Results: A rise in the thickness of the epidermis as well as an appropriate maturation and protein expression (Loricrin, Fillagrin, E-Cadherin and Cytokeratins 5&6) were detected in treated RHE samples. In particular, the HT-based formulation was found to stimulate cell proliferation, as evidenced by the significant increase in Ki67 expression, which suggests the involvement of repair mechanisms, increasing epithelial regeneration and differentiation and improving the epidermal barrier effect. Furthermore, HT-based formulation showed a statistically significant anti-inflammatory activity by reducing both IL-1α and IL-8 release by RHE tissues, greater than the reference drug dexamethasone. Finally, excellent transcutaneous absorption values were found for HT, demonstrating how this new formulation increases the availability of the bioactive compound.
Conclusions: In light of these results, Fenolia® Eudermal Cream 15 could be an effective agent to counteract atopic dermatitis. Graphical abstract Safety and efficacy of hydroxytyrosol-based formulation on skin inflammation: in vitro evaluation on reconstructed human epidermis model.
Keywords: Anti-inflammatory effect; Atopic dermatitis; Histology and immunohistochemistry; Hydroxytyrosol; In vitro reconstructed human epidermis; Semi-solid formulation.
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures




References
-
- Napolitano M, Megna M, Patruno C, Gisondi P, Ayala F, Balato N. Adult atopic dermatitis: a review. G Ital Dermatol Venereol. 2016;151:403–411. - PubMed
-
- Katsarou A, Armenaka MC. Atopic dermatitis in older patients: particular points. J Eur Acad Dermatol Venereol. 2011;25:12–18. - PubMed
-
- Silvestre Salvador JF, Romero-Pérez D, Encabo-Duràn B. Atopic dermatitis in adults: a diagnostic challenge. J Investig Allergol Clin Immunol. 2017;27:78–88. - PubMed
-
- Dinulos JG, Trickett A, Crudele C. New science and treatment paradigms for atopic dermatitis. Curr Opin Pediatr. 2018;30(1):161–168. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

