Chemical On/Off Switching of Mechanically Planar Chirality and Chiral Anion Recognition in a [2]Rotaxane Molecular Shuttle
- PMID: 31129959
- PMCID: PMC6693800
- DOI: 10.1021/jacs.9b00941
Chemical On/Off Switching of Mechanically Planar Chirality and Chiral Anion Recognition in a [2]Rotaxane Molecular Shuttle
Abstract
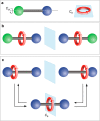
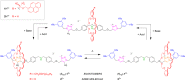
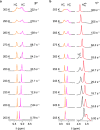
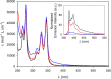
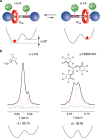
We exploit a reversible acid-base triggered molecular shuttling process to switch an appropriately designed rotaxane between prochiral and mechanically planar chiral forms. The mechanically planar enantiomers and their interconversion, arising from ring shuttling, have been characterized by NMR spectroscopy. We also show that the supramolecular interaction of the positively charged rotaxane with optically active anions causes an imbalance in the population of the two enantiomeric coconformations. This result represents an unprecedented example of chiral molecular recognition and can disclose innovative approaches to enantioselective sensing and catalysis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Sauvage J.-P.; Dietrich-Buchecker C.. Molecular Catenanes, Rotaxanes and Knots; Wiley: New York, 1999.
-
- Bruns C. J.; Stoddart J. F.. The Nature of the Mechanical Bond: From Molecules to Machines; Wiley: Hoboken, 2016.
-
- Balzani V.; Credi A.; Venturi M.. Molecular Devices and Machines - Concepts and Perspectives for the Nanoworld; Wiley-VCH: Weinheim, 2008.
Publication types
LinkOut - more resources
Full Text Sources

