Artificially Enhancing and Suppressing Hippocampus-Mediated Memories
- PMID: 31130452
- PMCID: PMC6548647
- DOI: 10.1016/j.cub.2019.04.065
Artificially Enhancing and Suppressing Hippocampus-Mediated Memories
Abstract
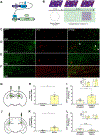
Emerging evidence indicates that distinct hippocampal domains differentially drive cognition and emotion [1, 2]; dorsal regions encode spatial, temporal, and contextual information [3-5], whereas ventral regions regulate stress responses [6], anxiety-related behaviors [7, 8], and emotional states [8-10]. Although previous studies demonstrate that optically manipulating cells in the dorsal hippocampus can drive the behavioral expression of positive and negative memories, it is unknown whether changes in cellular activity in the ventral hippocampus can drive such behaviors [11-14]. Investigating the extent to which distinct hippocampal memories across the longitudinal axis modulate behavior could aid in the understanding of stress-related psychiatric disorders known to affect emotion, memory, and cognition [15]. Here, we asked whether tagging and stimulating cells along the dorsoventral axis of the hippocampus could acutely, chronically, and differentially promote context-specific behaviors. Acute reactivation of both dorsal and ventral hippocampus cells that were previously active during memory formation drove freezing behavior, place avoidance, and place preference. Moreover, chronic stimulation of dorsal or ventral hippocampal fear memories produced a context-specific reduction or enhancement of fear responses, respectively, thus demonstrating bi-directional and context-specific modulation of memories along the longitudinal axis of the hippocampus. Fear memory suppression was associated with a reduction in hippocampal cells active during retrieval, while fear memory enhancement was associated with an increase in basolateral amygdala activity. Together, our data demonstrate that discrete sets of cells throughout the hippocampus provide key nodes sufficient to bi-directionally reprogram both the neural and behavioral expression of memory.
Keywords: emotion; engram; hippocampus; longitudinal axis; memory; optogenetics.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures



References
-
- Poppenk J, Evensmoen HR, Moscovitch M, and Nadel L (2013). Long-axis specialization of the human hippocampus. Trends in cognitive sciences 17, 230–240. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

