Nilotinib: A Tyrosine Kinase Inhibitor Mediates Resistance to Intracellular Mycobacterium Via Regulating Autophagy
- PMID: 31130711
- PMCID: PMC6562972
- DOI: 10.3390/cells8050506
Nilotinib: A Tyrosine Kinase Inhibitor Mediates Resistance to Intracellular Mycobacterium Via Regulating Autophagy
Abstract
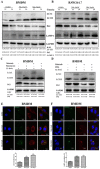
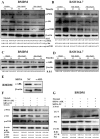
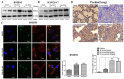
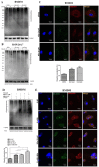
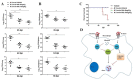
Nilotinib, a tyrosine kinase inhibitor, has been studied extensively in various tumor models; however, no information exists about the pharmacological action of nilotinib in bacterial infections. Mycobacterium bovis (M. bovis) and Mycobacterium avium subspecies paratuberculosis (MAP) are the etiological agents of bovine tuberculosis and Johne's disease, respectively. Although M. bovis and MAP cause distinct tissue tropism, both of them infect, reside, and replicate in mononuclear phagocytic cells of the infected host. Autophagy is an innate immune defense mechanism for the control of intracellular bacteria, regulated by diverse signaling pathways. Here we demonstrated that nilotinib significantly inhibited the intracellular survival and growth of M. bovis and MAP in macrophages by modulating host immune responses. We showed that nilotinib induced autophagic degradation of intracellular mycobacterium occurred via the inhibition of PI3k/Akt/mTOR axis mediated by abelson (c-ABL) tyrosine kinase. In addition, we observed that nilotinib promoted ubiquitin accumulation around M. bovis through activation of E3 ubiquitin ligase parkin. From in-vivo experiments, we found that nilotinib effectively controlled M. bovis growth and survival through enhanced parkin activity in infected mice. Altogether, our data showed that nilotinib regulates protective innate immune responses against intracellular mycobacterium, both in-vitro and in-vivo, and can be exploited as a novel therapeutic remedy for the control of M. bovis and MAP infections.
Keywords: Mycobacterium avium subspecies paratuberculosis (MAP); Mycobacterium bovis (M. bovis); autophagy; macrophage; nilotinib.
Conflict of interest statement
All authors read and approved the final manuscript. The authors declare that neither they nor their institutes have any competing conflict of interests.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

