Surgery of the lateral skull base: a 50-year endeavour
- PMID: 31130732
- PMCID: PMC6540636
- DOI: 10.14639/0392-100X-suppl.1-39-2019
Surgery of the lateral skull base: a 50-year endeavour
Abstract
Chirurgia della base del cranio laterale: 50 anni di impegno.
Riassunto: La base del cranio non è anatomicamente divisa in anteriore e laterale, ma è per semplicità che comunemente si intendono i corridoi chirurgici con direzione antero-laterale, laterale pura e postero laterale come “Approcci chirurgici della base del cranio laterale”. Una relazione con titolo “Cinquant’anni di impegno”, di sforzo o di dedizione, vuole essere il riconoscimento a questa chirurgia che nel corso degli anni ha sviluppato interventi sempre più complessi con una morbidità sempre minore. Il principio della chirurgia della base del cranio laterale si fonda sulla possibilità di “fare spazio”, esporre adeguatamente, rimuovere osso per salvaguardare il cervello, insieme alla possibilità di preservare la funzione e adattare l’approccio chirurgico all’istologia della lesione. Il concetto che l’istologia detta l’entità della resezione chirurgica, bilanciando la morbidità intrinseca di ciascun approccio, è oggetto di trattazione nella prima sezione di questa relazione. Nella seconda sezione sono descritti i principali approcci chirurgici, intesi non come descrizione tecnica di ciascun tempo chirurgico, ma dei principi che sono alla base di ciascun approccio. La terza sezione è dedicata alle questioni aperte, quelle ancora irrisolte, inerenti alcuni tumori ed il loro trattamento. L’argomento del neurinoma sporadico dell’ottavo nervo cranico è trattato riportando l'attuale dibattito sulla osservazione, la chirurgia di preservazione dell’udito, la riabilitazione con l’impianto cocleare, la radioterapia e le ricerche recenti su marcatori tumorali predittivi di crescita. Il paraganglioma del forame giugulare è trattato nel contesto della chirurgia radicale, chirurgia parziale, osservazione e radioterapia. La terapia dei meningiomi della base del cranio analizza il punto di vista specifico dell’otochirurgo e del neurochirurgo. Cordomi e condrosarcomi, tumori del sacco endolinfatico, carcinomi dell’orecchio e colesteatoma della rocca sono le altre lesioni affrontate. Infine, nella quarta sezione è proposto un contributo a libera scelta ad autori di riconosciuta esperienza. Lo scopo di questa relazione è stato quello di fornire un aggiornamento della chirurgia della base del cranio laterale dopo 50 anni di duro lavoro e, o forse soprattutto, di permettere alle tante questioni irrisolte, alle domande che ancora non hanno risposta, di trovare espressione, affinchè il dibattito ed il progresso possano continuare con la condivisione di esperienze. Se al termine della lettura vi saranno più domande che risposte, potremo dirci che l’obiettivo di questa relazione è stato raggiunto.
Keywords: Benign tumors of the skull base; Lateral approaches to the skull base; Lateral skull base surgery; Malignant tumors of the skull base; Skull base surgery.
Plain language summary
Disregarding the widely used division of skull base into anterior and lateral, since the skull base should be conceived as a single anatomic structure, it was to our convenience to group all those approaches that run from the antero-lateral, pure lateral and postero-lateral side of the skull base as “Surgery of the lateral skull base”. “50 years of endeavour” points to the great effort which has been made over the last decades, when more and more difficult surgeries were performed by reducing morbidity. The principle of lateral skull base surgery, “remove skull base bone to approach the base itself and the adjacent sites of the endo-esocranium”, was then combined with function preservation and with tailoring surgery to the pathology. The concept that histology dictates the extent of resection, balancing the intrinsic morbidity of each approach was the object of the first section of the present report. The main surgical approaches were described in the second section and were conceived not as a step-by-step description of technique, but as the highlighthening of the surgical principles. The third section was centered on open issues related to the tumor and its treatment. The topic of vestibular schwannoma was investigated with the current debate on observation, hearing preservation surgery, hearing rehabilitation, radiotherapy and the recent efforts to detect biological markers able to predict tumor growth. Jugular foramen paragangliomas were treated in the frame of radical or partial surgery, radiotherapy, partial “tailored” surgery and observation. Surgery on meningioma was debated from the point of view of the neurosurgeon and of the otologist. Endolymphatic sac tumors and malignant tumors of the external auditory canal were also treated, as well as chordomas, chondrosarcomas and petrous bone cholesteatomas. Finally, the fourth section focused on free-choice topics which were assigned to aknowledged experts. The aim of this work was attempting to report the state of the art of the lateral skull base surgery after 50 years of hard work and, above all, to raise questions on those issues which still need an answer, as to allow progress in knowledge through sharing of various experiences. At the end of the reading, if more doubts remain rather than certainties, the aim of this work will probably be achieved.
Figures

































References
2.2. Evidence-based therapy in diseases of the skull base
-
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ Sug 2003;73:712-8. - PubMed
-
- Schmoorl C, Gall C, Stampf S, et al. Correction of confounding bias in non-randomized studies by appropriate weighting. Biometrical J 2011;2:369-87. - PubMed
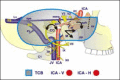
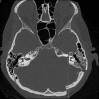
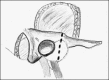
3.1. The translabyrinthine approach
-
- Fisch U, Mattox D. Microsurgery of the skullbase. Thieme Publishing Group; 1988.
-
- Friedman RA. Lateral skull base surgery. Thieme Publishing Group; 2012.
-
- Jackler RK. Atlas of skull base surgery and neurotology. Thieme Publishing Group; 2008.
-
- Sanna M. Atlas of microsurgery of the lateral skull base. Thieme Publishing Group; 2007.
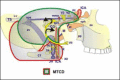
3.3. The modified transcochlear approaches
-
- House WF, Hitselberger WE. The transcochlear approach to the skull base. Arch Otolaryngol 1976;102:334-42. - PubMed
-
- Gantz BJ, Fisch U. Modified transotic approach to the cerebellopontile angle. Arch Otolaryngol 1983;109:252-6. - PubMed
-
- Sanna M, Mazzoni A, Saleh E, et al. The system of the modified transcochlear approach: a lateral avenue to the central skull base. Am J Otol 1998;19:88-97. - PubMed
-
- Sanna M, Saleh E, Krais T, et al. The transcochlear approaches. In: Atlas of microsurgery of the lateral skull base. Stuttgart, New York: Georg Thieme Verlag; 2008. pp. 80-129.
3.4. The middle cranial fossa approach
-
- House WF. Surgical exposure of the internal auditory canal and its contents through the middle, cranial fossa. Laryngoscope 1961;71:1363-85. - PubMed
-
- House WF, Shelton C. Middle fossa approach for acoustic tumor removal. Neurosurg Clin N Am 2008;19:279-88. - PubMed
-
- Fisch U, Yasargil G. Transtemporal extralabyrinthine operations on the internal auditory canal, the eighth and the seventh cranial nerves. In: Microsurgery applied to neurosurgery. Stuttgart, New York: Georg Thieme Verlag; 1969. pp. 195-210.
-
- Brackmann DE, House JR, Hitselberger WE. Technical modifications to the middle fossa craniotomy approach in removal of acoustic neuromas. Am J Otol 1994;15:614-9. - PubMed
-
- Wigand ME, Haid T, Berg M, et al. The enlarged transtemporal approach to the cerebellopontine angle: technique and indications. Acta Otorhinolaryngol Ital 1982;2:571-82.
3.5. The pterional approach
-
- Heuer GJ. Surgical experiences with an intracranial approach to chiasmal lesions. Arch Surg 1920;1:368-81.
-
- Yasargil MG, Fox JL. The microsurgical approach to intracranial aneurysms. Surg Neurol 1975;3:7-14. - PubMed
-
- Altay T, Couldwell WT. The frontotemporal (pterional) approach: an historical perspective. Neurosurg 2012;71:481-92. - PubMed
-
- Andrews RJ, Bringas JR. A review of brain retraction and recommendations for minimizing intraoperative brain injury. Neurosurg 1993;33:1052-63. - PubMed
-
- Spetzler RF, Daspit CP, Pappas CT. The combined supra- and infratentorial approach for lesions of the petrous and clival regions: experience with 46 cases. J Neurosurg 1992;76:588-99. - PubMed
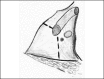
3.7. The petro-occipital transigmoid approach (POTS)
3.8. Presigmoid retrolabyrinthine approach and variation
-
- Ballance C. Some points in the surgery of the brain and its membranes. London: Macmillan and Co, Ltd.; 1907.
-
- Krause F. Zur Freilegung der hinteren felsenbeingflache und des kleinhirns. Beitrage Klin Chir 1903;37:728-64.
-
- Dandy WE. Removal of cerebellopontile (acoustic) tumors through a unilateral approach. Arch Surg 1934;29:3:337.
-
- Dandy WE. Results of removal of acoustic tumors by the unilateral approach. Arch Surg 1941;42:1026.
-
- McKenzie KG, Alexander E., Jr Acoustic neuroma. Clin Neurosurg 1954;2:21-36. - PubMed
3.9. Occipital approaches, retrosigmoid approach
-
- Mazzoni A, Zanoletti E, Denaro L, et al. Retrolabyrinthine meatotomy as part of retrosigmoid approach to expose the whole internal auditory canal: rationale, technique and outcome in hearing preservation surgery for vestibular schwannoma. Operat Neurosurgery 2018;14:36-44. - PubMed
3.10. The suprameatal approach and the transpetrous-transapex approach
-
- Samii M, Tatagiba M, Carvalho GA. Retrosigmoid intradural suprameatal approach to Meckel’s cave and the middle fossa: surgical technique and outcome. J Neurosurg 2000;92:235-41. - PubMed
-
- Seoane E, Rhoton AL. Suprameatal extension of the retrosigmoid approach: microsurgical anatomy. Neurosurgery 1999;44:553-60. - PubMed
-
- Rhoton AL., Jr The cerebellopontine angle and posterior fossa cranial nerves by the retrosigmoid approach. Neurosurgery 2000;47:S93-129. - PubMed
-
- Rigante L, Herlan S, Tatagiba MS, et al. Petrosectomy and topographical anatomy in traditional kawase and posterior intradural petrous apicectomy (pipa) approach: an anatomical study. World Neurosurg 2016;86:93-102. - PubMed
-
- Ebner FH, Koerbel A, Kirschniak A, et al. Endoscope-assisted retrosigmoid intradural suprameatal approach to the middle fossa: anatomical and surgical considerations. EJSO 2007;33:109-13. - PubMed
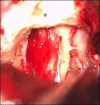
3.11. The extreme lateral approach: highlights on the key steps of surgical technique
-
- Kawashima M, Tanriover N, Rhoton AL, et al. Comparison of the far lateral and extreme lateral variants of the atlanto-occipital transarticular approach to anterior extradural lesions of the craniovertebral junction. Neurosurgery 2003;53:662-74. - PubMed
-
- Hadley MN, Spetzler RF, Sonntag VK. The transoral approach to the superior cervical spine. A review of 53 cases of extradural cervicomedullary compression. J Neurosurg 1989;71:16-23. - PubMed
-
- Samii M, Klekamp J, Carvalho G. Surgical results for meningiomas of the craniocervical junction. Neurosurgery 1996;39:1086-94. - PubMed
-
- Sen CN, Sekhar LN. An extreme lateral approach to intradural lesions of the cervical spine and foramen magnum. Neurosurgery 1990;27:197-204. - PubMed
-
- Babu RP, Sekhar LN, Wright DC. Extreme lateral transcondylar approach: technical improvements and lessons learned. J Neurosurg 1994;81:49-59. - PubMed
3.12. En bloc resections of the temporal bone
-
- Mazzoni A, Zanoletti E, Marioni G, et al. En bloc temporal bone resections in squamous cell carcinoma of the ear. technique, principles and limits. Acta Otolaryngol 2016;136:425-32. - PubMed
-
- Zanoletti E, Marioni G, Franchella S, et al. Recurrent squamous cell carcinoma of the temporal bone: critical analysis of cases with a poor prognosis. Am J Otolaryngol 2015;36:352-5. - PubMed
3.13. Totally endoscopic and combined endo-microscopic approaches in lateral skull base surgery
-
- Bennett M, Haynes DS. Surgical approaches and complications in the removal of vestibular schwannomas. Otolaryngol Clin North Am 2007;40:589-609. - PubMed
-
- Koos WT, Day JD, Matula C, et al. Neurotopographic considerations in the microsurgical treatment of small acoustic neurinomas. J Neurosurg 1998;88:506-12. - PubMed
-
- Marchioni D, Alicandri-Ciufelli M, Rubini A, et al. Endoscopic transcanal corridors to the lateral skull base: initial experiences. Laryngoscope 2015;125:S1-13. - PubMed
-
- Marchioni D, Bonali M, Presutti L. Transcanal endoscopic lateral skull base surgery. Operative Techniques in Otolaryngology-Head and Neck Surgery 2017;28:57-64.
-
- Marchioni D, Carner M, Soloperto D, et al. Expanded transcanal transpromontorial approach: a novel surgical technique for cerebellopontine angle vestibular schwannoma removal. Otolaryngol Head Neck Surg 2018;158:710-5. - PubMed
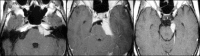
4.1.1. Observation in vestibular schwannomas - a systematic review
-
- xCarlson ML, Link MJ, Wanna GB, et al. Management of sporadic vestibular schwannoma. Otolaryngol Clin North Am 2015;48:407-22. - PubMed
-
- Carlson ML, Habermann EB, Wagie AE, et al. The changing landscape of vestibular schwannoma management in the united states - a shift toward conservatism. Otolaryngol Head Neck Surg 2015;153:440-6. - PubMed
-
- Stangerup S-E, Caye-Thomasen P. Epidemiology and natural history of vestibular schwannomas. Otolaryngol Clin North Am 2012;45:257-68. - PubMed
-
- Stangerup S-E, Caye-Thomasen P, Tos M, et al. The natural history of vestibular schwannoma. Otol Neurotol 2006;27:547-52. - PubMed
-
- American Academy of Otolaryngology-Head and Neck Surgery Foundation, INC. Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma). Otolaryngol Head Neck Surg 1995;113:179-80. - PubMed
4.1.2. Radiotherapy in acoustic neuroma
-
- Lin D, Hegarty JL, Fischbein NJ, et al. The prevalence of “incidental” acoustic neuroma. Arch Otolaryngol Head Neck Surg 2005;131:241-4. - PubMed
-
- Stangerup R, Tos M, Thomsen J, et al. True incidence of vestibular schwannoma? Neurosurgery 2010;67:1335-40. - PubMed
-
- Kirchmann M, Karnov K, Hansen S, et al. Ten-year follow-up on tumor growth and hearing in patients observed with an intracanalicular vestibular schwannoma. Neurosurgery 2017;80:49-56. - PubMed
-
- Krengli M, Zanoletti E, Deantonio L. Radiation therapy in acoustic neuroma. Wenz F. (editor). Radiation oncology. Springer International Publishing AG; 2018. 10.1007/978-3-319-52619-5_11-1. - DOI
-
- Berkowitz O, Han YY, Talbott EO, et al. Gamma knife radiosurgery for vestibular schwannomas and quality of life evaluation. Stereotact Funct Neurosurg 2017;95:166-73. - PubMed
4.1.3. Vestibular schwannoma: surgery after radiotherapy
-
- Stangerup SE, Caye-Thomasen P, Tos M, et al. The natural history of vestibular schwannoma. Otol Neurotol 2006;27:547-52. - PubMed
-
- Leksell L. A note on the treatment of acoustic tumors. Acta Chir Scand 1971;137:763-5. - PubMed
-
- Pollock BE. Management of vestibular schwannomas that enlarge after stereotactic radiosurgery: treatment recommendations based on a 15- year experience. Neurosurgery 2006;58:241-8. - PubMed
-
- Schulder M, Sreepada GS, Kwartler JA, et al. Microsurgical removal of a vestibular schwannoma after stereotactic radiosurgery: surgical and pathologic findings. Am J Otol 1999;20:364-7. - PubMed
-
- Patnaik U, Prasad SC, Tutar H, et al. The long-term outcomes of wait-and-scan and the role of radiotherapy in the management of vestibular schwannomas. Otol Neurotol 2015;36:638-46. - PubMed
4.1.4. Complications in acoustic neuroma surgery
-
- Waldman EH, Lustig LR. Sir Charles Alfred Ballance: contributions to otology and neurotology. Otol Neurotol 2005;26:1073-82. - PubMed
-
- Thomsen J, Tos M, Harmsen A, et al. Surgery of acoustic neuromas. Preliminary experience with a translabyrinthine approach. Acta Neurol Scand 1977;56:277-90. - PubMed
-
- Samii M, Matthies C. Management of 1,000 vestibular schwannomas (acoustic neuromas): surgical management and results with an emphasis on complications and how to avoid them. Neurosurg 1997;40:11-23. - PubMed
-
- Huang X, Xu M, Xu J, et al. Complications and management of large intracranial vestibular schwannomas via the retrosigmoid approach. World Neurosurg 2017;99:326-35. - PubMed
4.1.5. Hearing Preservation Surgery (HPS) with the retrosigmoid approach
-
- Elliot FA, McKissock W. Acoustic neuroma. Early diagnosis. Lancet 1954;2:1189-91. - PubMed
-
- Pertuiset B. La conservation des fonctions auditives et faciales au cours de l’exerèse totale des neurinomes de l’acoustique par voie sousoccipitale. Presse Med 1966;74:2327-30. - PubMed
-
- Smith MFW, Miller RN, Cox DJ. Suboccipital microsurgical removal of acoustic neuromas of all sizes. Ann Otol Rhinol Laryngol 1973;82:407-11. - PubMed
-
- Bremond G, Garcin M, Magnan J. Preservation of hearing in the removal of acoustic neuroma (“minima” posterior approach by retrosigmoid route). J Larygol Otol 1980;94:1199-204. - PubMed
-
- Sterkers JM, Corlieu P, Hamancl KF, et al. Chirurgie des tumeurs de l’acoustique par voie retosigmoide. Ann Otolaryng Ch Cervicofac 1980;97:519-32. - PubMed
4.1.6. Assessing hearing to orient the choice of treatment for acoustic neuroma
-
- Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): hearing function in 1000 tumor resections. Neurosurgery 1997;40:248-60. - PubMed
4.1.7. Vestibular schwannomas and cochlear implant
-
- Tveiten OV, Carlson ML, Goplen F, et al. Long-term auditory symptoms in patients with sporadic vestibular schwannoma: an international crosssectional study. Neurosurgery 2015;77:218-27. - PubMed
-
- Rabelo de Freitas M, Russo A, Sequino G, et al. Analysis of hearing preservation and facial nerve function for patients undergoing vestibular schwannoma surgery: the middle cranial fossa approach versus the retrosigmoid approach - personal experience and literature review. Audiol Neurotol 2012;17:71-81. - PubMed
-
- Sanna M, Khrais T, Russo A, et al. Hearing preservation surgery in vestibular schwannoma: the hidden truth. Ann Otol Laryngol 2004;113:156-63. - PubMed
-
- Lassaletta L, Aristegui M, Medina M, et al. Ipsilateral cochlear implantation in patients with sporadic vestibular schwannoma in the only or best ear and in patients with NF2. Eur Arch Otorhinolaryngol 2016;273:27-35. - PubMed
-
- Hoffman RA, Kohan D, Cohen NL. Cochlear implants in the management of bilateral acoustic neuromas. Am J Otol 1992;13:525-8. - PubMed
4.1.8. The hearing-focused therapy in acoustic neuroma: hearing preservation surgery, hearing rehabilitation with CI, observation
-
- Kanzaki J, Tos M, Sanna M, et al. New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma. Otol Neurotol 2003;24:642-8. - PubMed
-
- Kirchmann M, Karnov K, Hansen S, et al. Ten-year follow-up on tumor growth and hearing in patients observed with an intracanalicular vestibular schwannoma. Neurosurgery 2017;80:49-56. - PubMed
-
- Carlson ML, Jacob JT, Pollock BE, et al. Long-term hearing outcomes following stereotactic radiosurgery for vestibular schwannoma: patterns of hearing loss and variables influencing audiometric decline. J Neurosurg 2013;118:579-87. - PubMed
-
- Carlson ML, Link MJ, Wanna GB, et al. Management of sporadic vestibular schwannoma. Otolaryngol Clin North Am 2015;48:407-22. - PubMed
4.1.9. Current molecular knowledge on sporadic VIII cranial nerve schwannoma
-
- Lees KA, Tombers NM, Link MJ, et al. Natural history of sporadic vestibular schwannoma: a volumetric study of tumor growth. Otolaryngol Head Neck Surg 2018;159:535-42. - PubMed
-
- Martini A, Marioni G, Zanoletti E, et al. YAP, TAZ and AREG expression in eighth cranial nerve schwannoma. Int J Biol Markers 2017;32:e319-24. - PubMed
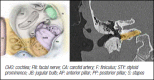
4.2.1. Treatment options for sporadic tympano-jugular paraganglioma (TJPGL)
-
- Sanna M, Jain Y, De Donato G, et al. Management of jugular paraganglioma: the Gruppo Otologico experience. Otol Neurotol 2004;25:797-804. - PubMed
-
- Gilbo P, Morris CG, Werning JW, et al. Radiotherapy for benign head and neck paraganglioma. A 45 years experience. Cancer 2014;20:3738-43. - PubMed
-
- Willem SN, Einstein DB, Maciunas RJ, et al. Treatment of glomus jugulare tumors in patients with advanced age. Planned limited resection followed by gamma knife radiosurgery. Otol Neurotol 2005:26:1229-34. - PubMed
-
- Harrison LB, Session RB, Hong WK. Head and neck cancer. a multidisciplinary approach. Philadelphia: Lippincot Williams & Wilkins; 2009. pp. 655-989.
4.2.2. Radical surgery in jugular foramen paragangliomas: indications and results
-
- Fisch U, Mattox D. Microsurgery of the Skull Base. Stuttgart, New York: Georg Thieme Verlag; 1988.
-
- Jansen JC, van den Berg R, Kuiper A, et al. Estimation of growth rate in patients with head and neck paragangliomas influences the treatment proposal. Cancer 2000;88:2811-6. - PubMed
-
- Prasad SC, Mimoune HA, D’Orazio F, et al. The role of wait-and-scan and the efficacy of radiotherapy in the treatment of temporal bone paragangliomas. Otol Neurotol 2014;35:922-31. - PubMed
-
- Carlson ML, Sweeney AD, Wanna GB, et al. Natural history of glomus jugulare: a review of 16 tumors managed with primary observation. Otolaryngol Head Neck Surg 2015;152:98-105. - PubMed
-
- Lope Ahmad RA, Sivalingam S, Konishi M, et al. Oncologic outcome in surgical management of jugular paraganglioma and factors influencing outcomes. Head Neck 2013;35:527-34. - PubMed
4.2.3. Primary radiotherapy in paraganglioma: indications and results
-
- Lee JH, Barich F, Karnell LH, et al. American College of Surgeons Commission on Cancer; American Cancer Society National cancer data base report on malignant paragangliomas of the head and neck. Cancer 2002;94:730-7. - PubMed
-
- Lee JA, Duh QY. Sporadic paraganglioma. World J Surg 2008;32:683. - PubMed
-
- Milia ME, Turri L, Beldì D, et al. Multidisciplinary approach in the treatment of malignant paraganglioma of the glomus vagale: a case report. Tumori 2011;97:225-8. - PubMed
-
- Trombetta M. The role of radiotherapy in the management of paraganglioma. Otorhinolaryngology Clinic: an International Journal 2011;3:25-9.
4.2.4. Partial surgery in paraganglioma: indications and results
-
- Sanna M, Jain Y, De Donato G, et al. Management of jugular paragangliomas: the Gruppo Otologico experience. Otol Neurotol 2004;25:797-804. - PubMed
-
- Oldring D, Fisch U. Glomus tumors of the temporal region: surgical therapy. Am J Otol 1979;1:7-18. - PubMed
-
- Pensak ML, Jackler RK. Removal of jugular foramen tumors: the fallopian bridge technique. Otolaryngol Head Neck Surgery 1997;117:586-91. - PubMed
-
- Sanna M, Shun SH, Piazza P, et al. Infratemporal fossa approach type a with transcondylar-transtubercular extension for Fisch type C2 to C4 tympanojugular paragangliomas. Head Neck 2014;36:1581-8. - PubMed
-
- Oker N, Tran Ba Huy P. Authors’ response to the letter on the article: “Malignant head/neck paragangliomas. Comparative study”. Eur Ann Otorhinolaryngol Head Neck Dis 2015;132:111. - PubMed
4.2.5. Management of internal carotid artery in skull base paraganglioma surgery
-
- Fisch U, Mattox D. Paragangliomas of the temporal bone. In: Micro-surgery of the skull base. Stuttgart, New York: Georg Thieme Verlag; 1988. pp. 148-281.
-
- Sanna M, Piazza P, Shin SH, et al. Microsurgery of skull base paragangliomas. Stuttgart, New York: Georg Thieme Verlag; 2013.
-
- Sanna M, Khrais T, Menozi R, et al. Surgical removal of jugular paragangliomas after stenting of the intratemporal internal carotid artery: a preliminary report. Laryngoscope 2006;116:742-6. - PubMed
-
- Piazza P, Di Lella F, Bacciu A, et al. Preoperative protectivestenting of the internal carotid artery in the management of complex head and neck paragangliomas: long-term results. Audiol Neurotol 2013;18:345-52. - PubMed
-
- Sanna M, Piazza P, Ditrapani G, et al. Management of the internal carotid artery in tumors of the lateral skull base: preoperative permanent balloon occlusion without reconstruction. Otol Neurotol 2004;25:998-1005. - PubMed
4.3.1 Chordoma and chondrosarcoma
-
- Borba LAB, Al-Mefty O, Mrak RE, et al. Cranial chordomas in children and adolescents. J Neurosurg 1996;84:584-91. - PubMed
-
- McMaster ML, Goldstein AM, Bromley CM, et al. Chordoma: incidence and survival patterns in the United States, 1973-1995. Cancer Causes Control 2001;12:1-11. - PubMed
-
- Horten BC, Montague SR. In vitro characteristics of a sacrococcygeal chordoma maintained in tissue and organ culture systems. Acta Neuropathol 1976;35:13-25. - PubMed
-
- Jahangiri A, Jian B, Miller L, et al. Skull base chordomas. Clinical features, prognostic factors, and therapeutics. Neurosurg Clin N Am 2013;24:79-88. - PubMed
4.3.2. Endolymphatic sac tumours
-
- Hassard AD, Boudreau SF, Cron CC. Adenoma of the endolymphatic sac. J Otolaryngol 1984;13:213-6. - PubMed
-
- Heffner DK. Low-grade adenocarcinoma of probable endolymphatic sac origin. A clinicopathologic study of 20 cases. Cancer 1989;64:2292-302. - PubMed
-
- Li JC, Brackmann DE, Lo WW, et al. Reclassification of aggressive adenomatous mastoid neoplasms as endolymphatic sac tumors. Laryngoscope 1993;103:1342-8. - PubMed
-
- Dornbos D, Kim HJ, Butman JA, et al. Review of the neurological implications of von Hippel-Lindau disease. JAMA Neurol 2018;75:620-7. - PubMed
4.3.3. Posterior fossa meningiomas: the neuro-otologist perspective
-
- Castellano F, Ruggiero G. Meningiomas of the posterior fossa. Acta Radiol Suppl 1953;104:1-177. - PubMed
-
- Martinez R, Vaquero J, Areitio E, et al. Meningiomas of the posterior fossa. Surg Neurol 1983;19:237-43. - PubMed
-
- Thomas NW, King TT. Meningiomas of the cerebellopontine angle. A report of 41 cases. Br J Neurosurg 1996;10:59-68. - PubMed
4.3.4. Lateral skull base meningiomas: the neurosurgeon’s perspective
-
- Philippon J, Cornu P. The recurrence of meningiomas. Al Mefty O. (editor). Meningiomas. New York, NY: Raven Press; 1991. pp. 87-106.
-
- Roser F, Nakamura M, Jacobs C, et al. Sphenoid wing meningiomas with osseous involvement. Surg Neurol 2005;64:37-43. - PubMed
-
- Russel SM, Benjamin V. Medial sphenoid ridge meningiomas: classification, microsurgical anatomy, operative nuances and long-term surgical outcome in 35 consecutive patients. Neurosurgery 2008;62(Suppl 1):38-50. - PubMed
-
- Bassiouni H, Asgari S, Sandalcioglu IE, et al. Anterior clinoidal meningiomas: functional out come after microsurgical resection in a consecutive series of 106 patients: clinical article. J Neurosurg 2009;111:1078-90. - PubMed
-
- Pamir MN, Belirgen M, Ozduman K, et al. Anterior clinoidal meningiomas: analysis of 43 consecutive surgically treated cases. Acta Neurochir (Wien) 2008;150:625-35. - PubMed
4.3.5. Primary squamous cell carcinoma of the temporal bone
-
- Lionello M, Stritoni P, Facciolo MC, et al. Temporal bone carcinoma. Current diagnostic, therapeutic, and prognostic concepts. J Surg Oncol 2014;110:383-92. - PubMed
-
- Gidley PW, DeMonte F. Temporal bone malignancies. Neurosurg Clin N Am 2013;24:97-110. - PubMed
-
- Sasaki CT. Distant metastases from ear and temporal bone cancer. ORL J Otorhinolaryngol Relat Spec 2001;63:250-1. - PubMed
-
- Zanoletti E, Lovato A, Stritoni P, et al. A critical look at persistent problems in the diagnosis, staging and treatment of temporal bone carcinoma. Cancer Treat Rev 2015;41:821-6. - PubMed
4.3.6. Petrous bone cholesteatoma
-
- Vashishth A, Singh Nagar TR, Mandal S, et al. Extensive intratemporal cholesteatomas: presentation, complications and surgical outcomes. Eur Arch Otorhinolaryngol 2015;272:289-95. - PubMed
-
- Tutar H, Goksu N, Aydil U, et al. An analysis of petrous bone cholesteatomas treated with translabyrinthine transotic petrosectomy. Acta Otolaryngol 2013;133:1053-7. - PubMed
-
- Sanna M, Pandya Y, Mancini F, et al. Petrous bone cholesteatoma: classification, management and review of the literature. Audiol Neurootol 2011;16:124-36. - PubMed
-
- Danesi G, Cooper T, Panciera DT, et al. Sanna classification and prognosis of cholesteatoma of the petrous part of the temporal bone: a retrospective series of 81 patients. Otol Neurotol 2016;37:787-92. - PubMed
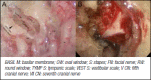
5.1. Petrous apex and surrounding areas lesions: clinical and surgical management
-
- Sanna M, Saleh E, Khrais T, et al. Atlas of microsurgery of the lateral skull base. Stuttgart, New York: Georg Thieme Verlag; 2008.
-
- Sanna M, Russo A, Taibah A, et al. The temporal bone: anatomical dissection and surgical approaches. Stuttgart, New York: Georg Thieme Verlag; 2018.
-
- Connor SE, Leung R, Natas S. Imaging of the petrous apex: a pictorial review. Br J Radiol 2008;81:427-35. - PubMed
-
- Razek AA, Huang BY. Lesions of the petrous apex: classification and findings at CT and MR imaging. Radiographics 2012;32:151-73. - PubMed
-
- Jackler RK, Cho M. A new theory to explain the genesis of petrous apex cholesterol granuloma. Otol Neurotol 2003;24:96-106. - PubMed
5.2. The evolving evidence based algorithm in vestibular schwannoma management
-
- Pirsig W, Ziermann-Becker B, Teschler-Nicola M. Acoustic neurofibromatosis in a child from the early bronze age. Tos M, Thomsen J. (editors). Proceedings of the first international conference on acoustic neuroma. Amsterdam, New York: Kugler Publications; 1992. pp. 7-12.
-
- Sandifort E. De duram quodam corpusculo, nervo auditorio adherente. In: Van de Eyk P, Vygh D. (eds). Observationes anatomicae-pathologicae. 1777;116-20.
-
- Bell C. The nervous system of the human body; embracing the papers delivered to the Royal Society on the subject of nerves. Appendix of cases. Washington DC: Duff Green; 1833. pp. 112-4.
-
- Ramsden RT. “A brilliant surgical result, the first recorded”: Anandale’s case, 3rd May 1895. J Laryngol Otol 1995;109:369-73. - PubMed
-
- Jerger J. Hearing tests in otologic diagnosis. Am Speech Hearing Assoc 1962;4:139-45.
5.3. Management of NF2: from vestibular schwannoma microsurgery to hearing restoration
-
- Evans DG, Moran A, King A, et al. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol 2005;26:93-7. - PubMed
-
- Lloyd SK, Evans DG. Neurofibromatosis type 2 service delivery in England. Neurochirurgie 2018;64:375-80. - PubMed
-
- Peyre M, Goutagny S, Bah A, et al. Conservative management of bilateral vestibular schwannomas in neurofibromatosis type 2 patients, hearing and tumor growth results. Neurosurgery 2013;72:907-13. - PubMed
-
- Fisher LM, Doherty JK, Lev MH, et al. Concordance of bilateral vestibular schwannoma growth and hearing changes in neurofibromatosis 2, neurofibromatosis 2 natural history consortium. Otol Neurotol 2009;30:835-41. - PubMed
5.4. When preservation of auditory function is a must: technique and outcome in a series of neurofibromatosis type II
-
- Mazzoni A, Zanoletti E, Denaro L, et al. Retrolabyrinthine meatotomy as part of retrosigmoid approach to expose the whole internal auditory canal: rationale, technique, and outcome in hearing preservation surgery for vestibular schwannoma. Oper Neurosurg (Hagerstown) 2018;14:36-44. - PubMed
-
- Samii M, Matthies C. Management of 1,000 vestibular schwannomas (acoustic neuromas): hearing function in 1,000 tumor resections. Neurosurgery 1997;40:248-60. - PubMed
-
- Samii M, Matthies C, Tatagiba M. Management of vestibular schwannomas (acoustic neuromas): auditory and facial nerve function after resection of 120 vestibular schwannomas in patients with neurofibromatosis 2. Neurosurgery 1997;40:696-705. - PubMed
-
- Samii M, Gerganov V, Samii A. Improved preservation of hearing and facial nerve function in vestibular schwannoma surgery via the retrosigmoid approach in a series of 200 patients. JNS 2006;105:527-35. - PubMed
-
- Samii M, Gerganov VM, Samii A. Microsurgery management of vestibular schwannomas in neurofibromatosis type 2: indications and results. Prog Neurol Surg 2008;21:169-75. - PubMed
5.5. Endoscope-assisted microsurgery of trigeminal, facial and auditory nerves
-
- Barrow D. Surgery of the cranials nerves of the posterior fossa. AANS Publications;1993.
-
- Dandy W. Concerning the cause of trigeminal neuralgia. Am J Surg 1934;24:447-55.
-
- Gardner J. Concerning the mechanism of trigeminal neuralgia and hemifacial spasm. J Neurosurg 1962;19:947-58. - PubMed
-
- Jannetta P. Trigeminal neuralgia and hemifacial spasm-etiology and definitive treatment. Trans Am Neurol Assoc 1975;100:89-91. - PubMed
-
- Bremond G, Garcin M, Magnan J, et al. L’abord “a minima” de l’espace pontocerebelleux. Cah ORL 1974;9:443-60.
5.6. Modern shifts in the clinical epidemiology of sporadic vestibular schwannoma and its implications
-
- Cushing H. Tumors of the nervus acusticus and the syndrome of the cerebellopontile ange. Philadelphia and London: WB Saunders Company; 1917. p. 291.
-
- Carlson ML, Habermann EB, Wagie AE, et al. The changing landscape of vestibular schwannoma management in the United States. A shift toward conservatism. Otolaryngol Head Neck Surg 2015;153:440-6. - PubMed
5.7. Preventing surgical morbidity in jugular paraganglioma
-
- Mazzoni A, Zanoletti E. Observation and partial targeted surgery in the management of tympano-jugular paraganglioma: a contribution to the multioptional treatment. Eur Arch Otorhinolaryngol 2016;273:635-42. - PubMed
-
- Mazzoni A, Cazzador D, d’Avella D, et al. Large intradural growth of tympano-jugular paraganglioma: a contribution to surgery and management. World Neurosurg 2019;122:e1482-90. - PubMed

