5-HT1A Receptor Agonist Promotes Retinal Ganglion Cell Function by Inhibiting OFF-Type Presynaptic Glutamatergic Activity in a Chronic Glaucoma Model
- PMID: 31130845
- PMCID: PMC6509153
- DOI: 10.3389/fncel.2019.00167
5-HT1A Receptor Agonist Promotes Retinal Ganglion Cell Function by Inhibiting OFF-Type Presynaptic Glutamatergic Activity in a Chronic Glaucoma Model
Abstract
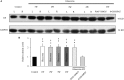
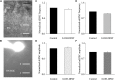
Serotonin receptors are potential neuroprotective agents in degenerative diseases of the central nervous system. The protective effects of serotonin receptor (5-HT1A) agonists on the survival and function of retinal ganglion cells (RGCs) by regulating the release of the presynaptic neurotransmitter γ-aminobutyric acid (GABA) were confirmed in our previous study of a chronic glaucoma rat model. However, the roles of excitatory amino acids and their interactions with the 5-HT1A receptor in glaucoma remain unknown. Here, we found that ocular hypertension increased glutamine synthetase (GS) and excitatory amino acid transporter 2 (EAAT2) expression in rat retinas. In addition, the high expression of GS and EAAT2 induced by glaucoma was downregulated by the 5-HT1A receptor agonist 8-OH-DPAT and the 5-HT1A receptor antagonist WAY-100635, respectively. Patch-clamp techniques were used to record glutamate receptor-mediated spontaneous and miniature glutamatergic excitatory post-synaptic currents (sEPSCs and mEPSCs) as well as L-glutamate-induced current in OFF-type and ON-type RGCs in rat retinal slices. Although there were no significant differences in the frequency and amplitude of sEPSC and mEPSC release between normal and glaucoma OFF- and ON-type RGCs, exogenous 8-OH-DPAT administration specifically reduced the frequency, but not the amplitude, of sEPSC and mEPSC release in glaucoma OFF-type rather than ON-type RGCs; these effects were completely blocked by WAY-100635. In summary, 8-OH-DPAT decreases and increases GS and EAAT2 expression of glaucomatous retina, respectively, while decreasing sEPSC and mEPSC frequency. In contrast, WAY-100635 increases and decreases GS and EAAT2 expression of glaucomatous retina, respectively, while increasing sEPSC and mEPSC frequency. The reduction of glutamatergic presynaptic transmission by 8-OH-DPAT deactivates RGCs at the neural network level and reduces the excitotoxic damage in the pathological process of chronic glaucoma.
Keywords: 5-HT1A receptor; OFF-type RGCs; glaucoma; glutamate release; neuroprotection.
Figures







References
LinkOut - more resources
Full Text Sources
Miscellaneous

