Functional Characterization of Dark Sleeper (Odontobutis obscura) TBK1 on IFN Regulation
- PMID: 31130963
- PMCID: PMC6510163
- DOI: 10.3389/fimmu.2019.00985
Functional Characterization of Dark Sleeper (Odontobutis obscura) TBK1 on IFN Regulation
Abstract
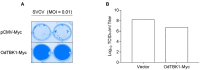
In East Asia, the dark sleeper, Odontobutis obscura (O. obscura) is a crucial commercial species of freshwater fish; however, its molecular biology research is still undeveloped, including its innate immune system, which is pivotal to antiviral responses. In this study, we cloned and identified the characterization and kinase function of dark sleeper TANK-binding kinase 1 (TBK1), supplementing the evidence of the conservation of this classical factor in fish. First, the ORF of Odontobutis obscurus (O. obscura) TBK1 (OdTBK1) was cloned from liver tissue by RACE-PCR. Subsequent nucleic acid and amino acid sequence analysis suggested that OdTBK1 is homologous with other fish TBK1, and the N-terminal Serine/Threonine protein kinases catalytic domain (S_TKc) and C-terminal coiled coil domain (CCD) are conserved. Subsequently, the cellular distribution demonstrated that OdTBK1 was located in the cytoplasm region. With regard to the identification of functions, OdTBK1 activated several interferon (IFN) promoters' activity and induced downstream IFN-stimulated genes (ISGs) expression. In a canonical manner, wild-type OdTBK1 significantly phosphorylated interferon regulatory factor 3 (IRF3) but failed when the N-terminal region was truncated. Furthermore, overexpression of OdTBK1 decreased viral proliferation remarkably. Collectively, these data systematically analyzed the characterization and function of OdTBK1, initiating the study of the innate antiviral response of dark sleeper.
Keywords: IFN; Odontobutis obscura; RLRs; TBK1; antiviral.
Figures







Similar articles
-
Functional characterization of dark sleeper (Odontobutis obscura) IRF3 in IFN regulation.Fish Shellfish Immunol. 2019 Jun;89:411-419. doi: 10.1016/j.fsi.2019.04.019. Epub 2019 Apr 10. Fish Shellfish Immunol. 2019. PMID: 30978449
-
Molecular characterization of a cyprinid fish (Ancherythroculter nigrocauda) TBK1 and its kinase activity in IFN regulation.Dev Comp Immunol. 2021 Jan;114:103805. doi: 10.1016/j.dci.2020.103805. Epub 2020 Aug 2. Dev Comp Immunol. 2021. PMID: 32755617
-
TBK1 from orange-spotted grouper exerts antiviral activity against fish viruses and regulates interferon response.Fish Shellfish Immunol. 2018 Feb;73:92-99. doi: 10.1016/j.fsi.2017.12.010. Epub 2017 Dec 6. Fish Shellfish Immunol. 2018. PMID: 29222027
-
The antiviral innate immune response in fish: evolution and conservation of the IFN system.J Mol Biol. 2013 Dec 13;425(24):4904-20. doi: 10.1016/j.jmb.2013.09.033. Epub 2013 Sep 27. J Mol Biol. 2013. PMID: 24075867 Review.
-
Immunity to betanodavirus infections of marine fish.Dev Comp Immunol. 2014 Apr;43(2):174-83. doi: 10.1016/j.dci.2013.07.019. Epub 2013 Aug 2. Dev Comp Immunol. 2014. PMID: 23916690 Review.
Cited by
-
Molecular Identification and Expression Analysis of NOD1/2 and TBK1 in Response to Viral or Bacterial Infection in the Spotted Knifejaw (Oplegnathus punctatus).Animals (Basel). 2025 Mar 31;15(7):1006. doi: 10.3390/ani15071006. Animals (Basel). 2025. PMID: 40218399 Free PMC article.
-
Two Duplicated Ptpn6 Homeologs Cooperatively and Negatively Regulate RLR-Mediated IFN Response in Hexaploid Gibel Carp.Front Immunol. 2021 Nov 26;12:780667. doi: 10.3389/fimmu.2021.780667. eCollection 2021. Front Immunol. 2021. PMID: 34899743 Free PMC article.
-
Genomic deciphering of sex determination and unique immune system of a potential model species rare minnow (Gobiocypris rarus).Sci Adv. 2022 Feb 4;8(5):eabl7253. doi: 10.1126/sciadv.abl7253. Epub 2022 Feb 2. Sci Adv. 2022. PMID: 35108042 Free PMC article.
-
Protein Phosphatase PP1 Negatively Regulates IRF3 in Response to GCRV Infection in Grass Carp (Ctenopharyngodon idella).Front Immunol. 2021 Jan 22;11:609890. doi: 10.3389/fimmu.2020.609890. eCollection 2020. Front Immunol. 2021. PMID: 33584687 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

