MASP-1 Increases Endothelial Permeability
- PMID: 31130964
- PMCID: PMC6509239
- DOI: 10.3389/fimmu.2019.00991
MASP-1 Increases Endothelial Permeability
Abstract
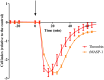
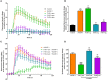
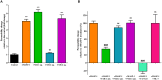
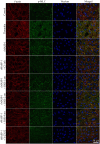
Pathologically increased vascular permeability is an important dysfunction in the pathomechanism of life-threatening conditions, such as sepsis, ischemia/reperfusion, or hereditary angioedema (HAE), diseases accompanied by uncontrolled activation of the complement system. HAE for example is caused by the deficiency of C1-inhibitor (the main regulator of early complement activation), which leads to edematous attacks threatening with circulatory collapse. We have previously reported that endothelial cells become activated during HAE attacks. A natural target of C1-inhibitor is mannan-binding lectin-associated serine protease-1 (MASP-1), a multifunctional serine protease, which plays a key role in the activation of complement lectin pathway. We have previously shown that MASP-1 induces the pro-inflammatory activation of endothelial cells and in this study we investigated whether MASP-1 can directly affect endothelial permeability. All experiments were performed on human umbilical vein endothelial cells (HUVECs). Real-time micro electric sensing revealed that MASP-1 decreases the impedance of HUVEC monolayers and in a recently developed permeability test (XperT), MASP-1 dose-dependently increased endothelial paracellular transport. We show that protease activated receptor-1 mediated intracellular Ca2+-mobilization, Rho-kinase activation dependent myosin light chain (MLC) phosphorylation, cytoskeletal actin rearrangement, and disruption of interendothelial junctions are underlying this phenomenon. Furthermore, in a whole-transcriptome microarray analysis MASP-1 significantly changed the expression of 25 permeability-related genes in HUVECs-for example it up-regulated bradykinin B2 receptor expression. According to our results, MASP-1 has potent permeability increasing effects. During infections or injuries MASP-1 may help eliminate the microbes and/or tissue debris by enhancing the extravasation of soluble and cellular components of the immune system, however, it may also play a role in the pathomechanism of diseases, where edema formation and complement lectin pathway activation are simultaneously present. Our findings also raise the possibility that MASP-1 may be a promising target of anti-edema drug development.
Keywords: C1-inhibitor; MASP-1; PAR-1; XPerT assay; angioedema; endothelial cell; permeability; transcriptome analysis.
Figures


References
-
- Héja D, Kocsis A, Dobó J, Szilágyi K, Szász R, Závodszky P, et al. . Revised mechanism of complement lectin-pathway activation revealing the role of serine protease MASP-1 as the exclusive activator of MASP-2. Proc Natl Acad Sci USA. (2012b) 109:10498–503. 10.1073/pnas.1202588109 - DOI - PMC - PubMed
-
- Megyeri M, Harmat V, Major B, Végh Á, Balczer J, Héja D, et al. . Quantitative characterization of the activation steps of mannan-binding lectin (MBL)-associated serine proteases (MASPs) points to the central role of MASP-1 in the initiation of the complement lectin pathway. J Biol Chem. (2013) 288:8922–34. 10.1074/jbc.M112.446500 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

