Bronchopulmonary Dysplasia: Crosstalk Between PPARγ, WNT/β-Catenin and TGF-β Pathways; The Potential Therapeutic Role of PPARγ Agonists
- PMID: 31131268
- PMCID: PMC6509750
- DOI: 10.3389/fped.2019.00176
Bronchopulmonary Dysplasia: Crosstalk Between PPARγ, WNT/β-Catenin and TGF-β Pathways; The Potential Therapeutic Role of PPARγ Agonists
Abstract
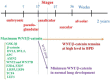
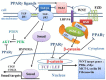
Bronchopulmonary dysplasia (BPD) is a serious pulmonary disease which occurs in preterm infants. Mortality remains high due to a lack of effective treatment, despite significant progress in neonatal resuscitation. In BPD, a persistently high level of canonical WNT/β-catenin pathway activity at the canalicular stage disturbs the pulmonary maturation at the saccular and alveolar stages. The excessive thickness of the alveolar wall impairs the normal diffusion of oxygen and carbon dioxide, leading to hypoxia. Transforming growth factor (TGF-β) up-regulates canonical WNT signaling and inhibits the peroxysome proliferator activated receptor gamma (PPARγ). This profile is observed in BPD, especially in animal models. Following a premature birth, hypoxia activates the canonical WNT/TGF-β axis at the expense of PPARγ. This gives rise to the differentiation of fibroblasts into myofibroblasts, which can lead to pulmonary fibrosis that impairs the respiratory function after birth, during childhood and even adulthood. Potential therapeutic treatment could target the inhibition of the canonical WNT/TGF-β pathway and the stimulation of PPARγ activity, in particular by the administration of nebulized PPARγ agonists.
Keywords: PPARγ; TGF-β; bronchopulmonary dysplasia; canonical WNT/β-catenin; fibrosis; myofibroblast; nebulized thiazolidinediones.
Figures

Similar articles
-
Crosstalk Between Peroxisome Proliferator-Activated Receptor Gamma and the Canonical WNT/β-Catenin Pathway in Chronic Inflammation and Oxidative Stress During Carcinogenesis.Front Immunol. 2018 Apr 13;9:745. doi: 10.3389/fimmu.2018.00745. eCollection 2018. Front Immunol. 2018. PMID: 29706964 Free PMC article. Review.
-
Hyperoxia-induced neonatal rat lung injury involves activation of TGF-{beta} and Wnt signaling and is protected by rosiglitazone.Am J Physiol Lung Cell Mol Physiol. 2009 Jun;296(6):L1031-41. doi: 10.1152/ajplung.90392.2008. Epub 2009 Mar 20. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19304912 Free PMC article.
-
Interactions between TGF-β1, canonical WNT/β-catenin pathway and PPAR γ in radiation-induced fibrosis.Oncotarget. 2017 Sep 23;8(52):90579-90604. doi: 10.18632/oncotarget.21234. eCollection 2017 Oct 27. Oncotarget. 2017. PMID: 29163854 Free PMC article. Review.
-
Necrotizing Enterocolitis: LPS/TLR4-Induced Crosstalk Between Canonical TGF-β/Wnt/β-Catenin Pathways and PPARγ.Front Pediatr. 2021 Oct 12;9:713344. doi: 10.3389/fped.2021.713344. eCollection 2021. Front Pediatr. 2021. PMID: 34712628 Free PMC article. Review.
-
Interplay between the renin-angiotensin system, the canonical WNT/β-catenin pathway and PPARγ in hypertension.Curr Hypertens Rep. 2018 Jun 9;20(7):62. doi: 10.1007/s11906-018-0860-4. Curr Hypertens Rep. 2018. PMID: 29884931 Review.
Cited by
-
CircRNA, lncRNA, and mRNA profiles of umbilical cord blood exosomes from preterm newborns showing bronchopulmonary dysplasia.Eur J Pediatr. 2022 Sep;181(9):3345-3365. doi: 10.1007/s00431-022-04544-2. Epub 2022 Jul 5. Eur J Pediatr. 2022. PMID: 35790551 Free PMC article.
-
Artesunate Alleviates Hyperoxia-Induced Lung Injury in Neonatal Mice by Inhibiting NLRP3 Inflammasome Activation.Evid Based Complement Alternat Med. 2023 Feb 4;2023:7603943. doi: 10.1155/2023/7603943. eCollection 2023. Evid Based Complement Alternat Med. 2023. PMID: 36785753 Free PMC article.
-
β-catenin activates TGF-β-induced epithelial-mesenchymal transition in adenomyosis.Exp Mol Med. 2020 Oct;52(10):1754-1765. doi: 10.1038/s12276-020-00514-6. Epub 2020 Oct 15. Exp Mol Med. 2020. PMID: 33060769 Free PMC article.
-
Modulatory Effects of Estradiol and Its Mixtures with Ligands of GPER and PPAR on MAPK and PI3K/Akt Signaling Pathways and Tumorigenic Factors in Mouse Testis Explants and Mouse Tumor Leydig Cells.Biomedicines. 2022 Jun 12;10(6):1390. doi: 10.3390/biomedicines10061390. Biomedicines. 2022. PMID: 35740412 Free PMC article.
-
Inhibition of retinal neovascularization by a PEDF-derived nonapeptide in newborn mice subjected to oxygen-induced ischemic retinopathy.Exp Eye Res. 2020 Jun;195:108030. doi: 10.1016/j.exer.2020.108030. Epub 2020 Apr 6. Exp Eye Res. 2020. PMID: 32272114 Free PMC article.
References
-
- Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. (1967) 276:357–68. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

