Preclinical evaluation of an integrated robotic system for magnetic resonance imaging guided shoulder arthrography
- PMID: 31131290
- PMCID: PMC6519665
- DOI: 10.1117/1.JMI.6.2.025006
Preclinical evaluation of an integrated robotic system for magnetic resonance imaging guided shoulder arthrography
Abstract
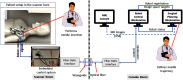
Shoulder arthrography is a diagnostic procedure which involves injecting a contrast agent into the joint space for enhanced visualization of anatomical structures. Typically, a contrast agent is injected under fluoroscopy or computed tomography (CT) guidance, resulting in exposure to ionizing radiation, which should be avoided especially in pediatric patients. The patient then waits for the next available magnetic resonance imaging (MRI) slot for obtaining high-resolution anatomical images for diagnosis, which can result in long procedure times. Performing the contrast agent injection under MRI guidance could overcome both these issues. However, it comes with the challenges of the MRI environment including high magnetic field strength, limited ergonomic patient access, and lack of real-time needle guidance. We present the development of an integrated robotic system to perform shoulder arthrography procedures under intraoperative MRI guidance, eliminating fluoroscopy/CT guidance and patient transportation from the fluoroscopy/CT room to the MRI suite. The average accuracy of the robotic manipulator in benchtop experiments is 0.90 mm and 1.04 deg, whereas the average accuracy of the integrated system in MRI phantom experiments is 1.92 mm and 1.28 deg at the needle tip. Based on the American Society for Testing and Materials (ASTM) tests performed, the system is classified as MR conditional.
Keywords: image-guided; magnetic resonance imaging; robot; shoulder arthrography.
Figures