A personalized platform identifies trametinib plus zoledronate for a patient with KRAS-mutant metastatic colorectal cancer
- PMID: 31131321
- PMCID: PMC6531007
- DOI: 10.1126/sciadv.aav6528
A personalized platform identifies trametinib plus zoledronate for a patient with KRAS-mutant metastatic colorectal cancer
Abstract
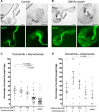
Colorectal cancer remains a leading source of cancer mortality worldwide. Initial response is often followed by emergent resistance that is poorly responsive to targeted therapies, reflecting currently undruggable cancer drivers such as KRAS and overall genomic complexity. Here, we report a novel approach to developing a personalized therapy for a patient with treatment-resistant metastatic KRAS-mutant colorectal cancer. An extensive genomic analysis of the tumor's genomic landscape identified nine key drivers. A transgenic model that altered orthologs of these nine genes in the Drosophila hindgut was developed; a robotics-based screen using this platform identified trametinib plus zoledronate as a candidate treatment combination. Treating the patient led to a significant response: Target and nontarget lesions displayed a strong partial response and remained stable for 11 months. By addressing a disease's genomic complexity, this personalized approach may provide an alternative treatment option for recalcitrant disease such as KRAS-mutant colorectal cancer.
Figures



References
-
- Izzedine H., Rixe O., Billemont B., Baumelou A., Deray G., Angiogenesis inhibitor therapies: Focus on kidney toxicity and hypertension. Am. J. Kidney Dis. 50, 203–218 (2007). - PubMed
-
- Røed Skårderud M., Polk A., Kjeldgaard Vistisen K., Larsen F. O., Nielsen D. L., Efficacy and safety of regorafenib in the treatment of metastatic colorectal cancer: A systematic review. Cancer Treat. Rev. 62, 61–73 (2018). - PubMed
-
- Chang Y. Y., Lin P. C., Lin H. H., Lin J. K., Chen W. S., Jiang J. K., Yang S. H., Liang W. Y., Chang S. C., Mutation spectra of RAS gene family in colorectal cancer. Am. J. Surg. 212, 537–544.e3 (2016). - PubMed
-
- Valtorta E., Misale S., Sartore-Bianchi A., Nagtegaal I. D., Paraf F., Lauricella C., Dimartino V., Hobor S., Jacobs B., Ercolani C., Lamba S., Scala E., Veronese S., Laurent-Puig P., Siena S., Tejpar S., Mottolese M., Punt C. J. A., Gambacorta M., Bardelli A., Di Nicolantonio F., KRAS gene amplification in colorectal cancer and impact on response to EGFR-targeted therapy. Int. J. Cancer 133, 1259–1265 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

