Cancer-associated mutation and beyond: The emerging biology of isocitrate dehydrogenases in human disease
- PMID: 31131326
- PMCID: PMC6530995
- DOI: 10.1126/sciadv.aaw4543
Cancer-associated mutation and beyond: The emerging biology of isocitrate dehydrogenases in human disease
Abstract
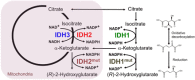
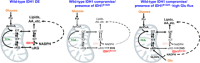
Isocitrate dehydrogenases (IDHs) are critical metabolic enzymes that catalyze the oxidative decarboxylation of isocitrate to α-ketoglutarate (αKG), NAD(P)H, and CO2. IDHs epigenetically control gene expression through effects on αKG-dependent dioxygenases, maintain redox balance and promote anaplerosis by providing cells with NADPH and precursor substrates for macromolecular synthesis, and regulate respiration and energy production through generation of NADH. Cancer-associated mutations in IDH1 and IDH2 represent one of the most comprehensively studied mechanisms of IDH pathogenic effect. Mutant enzymes produce (R)-2-hydroxyglutarate, which in turn inhibits αKG-dependent dioxygenase function, resulting in a global hypermethylation phenotype, increased tumor cell multipotency, and malignancy. Recent studies identified wild-type IDHs as critical regulators of normal organ physiology and, when transcriptionally induced or down-regulated, as contributing to cancer and neurodegeneration, respectively. We describe how mutant and wild-type enzymes contribute on molecular levels to disease pathogenesis, and discuss efforts to pharmacologically target IDH-controlled metabolic rewiring.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

