Identifying market risk for substandard and falsified medicines: an analytic framework based on qualitative research in China, Indonesia, Turkey and Romania
- PMID: 31131333
- PMCID: PMC6518437
- DOI: 10.12688/wellcomeopenres.15236.1
Identifying market risk for substandard and falsified medicines: an analytic framework based on qualitative research in China, Indonesia, Turkey and Romania
Abstract
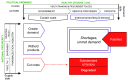
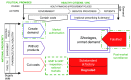
Introduction: Substandard and falsified medicines undermine health systems. We sought to unravel the political and economic factors which drive the production of these products, and to explain how they reach patients. Methods: We conducted in-depth case studies in China, Indonesia, Turkey and Romania. We reviewed academic papers and press reports (n = 840), developing semi-structured questionnaires. We interviewed regulators, policy-makers, pharmaceutical manufacturers, physicians, pharmacists, patients and academics (n=88). We coded data using NVivo software, and developed an analytic framework to assess national risks for substandard and falsified medicines. We tested the framework against cases reported to the World Health Organization, from countries at all income levels. Results: We found that increasing political commitment to provision of universal health coverage has led to public procurement policies aimed at lowering prices of medical products. In response, legitimate, profit-driven pharmaceutical companies protect their margins by cutting costs, or withdrawing from less profitable markets, while distributors engage in arbitrage. Meanwhile, health providers sometimes protect profits by 'upselling' patients to medicines not covered by insurers. Cost-cutting can undermine quality assurance, leading to substandard or degraded medicines. Other responses contribute to shortages, irrational demand and high prices. All of these provide market opportunities for producers of falsified products; they also push consumers outside of the regular supply chain, providing falsifiers with easy access to customers. The analytic framework capturing these interactions explained cases in most high and middle-income settings; additional factors operate in the poorest countries. Conclusions: Most efforts to secure medicine quality currently focus on product regulation. However, our research suggests market mechanisms are key drivers for poor quality medicines, including where political commitments to universal health coverage are under-resourced. We have developed a framework to guide country-specific, system-wide analysis. This can flag risks and pinpoint specific actions to protect medicine quality, and thus health.
Keywords: LMIC; counterifeit medicine; falsified medicine; medicine quality; procurement; substandard medicine.
Conflict of interest statement
Competing interests: In 2017, E.P. received consultancy fees from the World Health Organization for separate contributions to work on medicine quality. All other authors declare they have no conflict of interest.
Figures

References
-
- Review on Antimicrobial Resistance: Safe, Secure and Controlled: Managing the Supply Chain of Antimicrobials.London: AMR Review;2015. Reference Source
-
- Pisani E: Antimicrobial resistance: What does medicine quality have to do with it?London: Antimicrobial Review;2015. Reference Source
-
- World Health Organization: WHO Global Surveillance and Monitoring System for substandard and falsified medical products.Geneva: WHO; Report No.: WHO/EMP/RHT/2017.01.2017. Reference Source
-
- World Health Organization: A study on the public health and socioeconomic impact of substandard and falsified medical products.Geneva: WHO; Report No.: WHO/EMP/RHT/2017.02.2017. Reference Source
Grants and funding
LinkOut - more resources
Full Text Sources

