Population Pharmacokinetics of Alemtuzumab (Campath) in Pediatric Hematopoietic Cell Transplantation: Towards Individualized Dosing to Improve Outcome
- PMID: 31131436
- PMCID: PMC6885503
- DOI: 10.1007/s40262-019-00782-0
Population Pharmacokinetics of Alemtuzumab (Campath) in Pediatric Hematopoietic Cell Transplantation: Towards Individualized Dosing to Improve Outcome
Abstract
Background and objective: Alemtuzumab (Campath®) is used to prevent graft-versus-host disease and graft failure following pediatric allogeneic hematopoietic cell transplantation. The main toxicity includes delayed immune reconstitution, subsequent viral reactivations, and leukemia relapse. Exposure to alemtuzumab is highly variable upon empirical milligram/kilogram dosing.
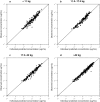
Methods: A population pharmacokinetic (PK) model for alemtuzumab was developed based on a total of 1146 concentration samples from 206 patients, aged 0.2-19 years, receiving a cumulative intravenous dose of 0.2-1.5 mg/kg, and treated between 2003 and 2015 in two centers.
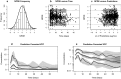
Results: Alemtuzumab PK were best described using a two-compartment model with a parallel saturable and linear elimination pathway. The linear clearance pathway, central volume of distribution, and intercompartmental distribution increased with body weight. Blood lymphocyte counts, a potential substrate for alemtuzumab, did not impact clearance.
Conclusion: The current practice with uniform milligram/kilogram doses leads to highly variable exposures in children due to the non-linear relationship between body weight and alemtuzumab PK. This model may be used for individualized dosing of alemtuzumab.
Conflict of interest statement
Rick Admiraal, Cornelia M. Jol-van der Zijde, Juliana M. Furtado Silva, Catherijne A.J. Knibbe, Arjan C. Lankester, Jaap Jan Boelens, Goeff Hale, Aniekan Etuk, Melanie Wilson, Stuart Adams, Paul Veys, Charlotte van Kesteren and Robbert G.M. Bredius have no conflicts of interest to declare.
Figures


References
-
- Marsh RA, Lane A, Mehta PA, et al. Alemtuzumab levels impact acute GVHD, mixed chimerism, and lymphocyte recovery following alemtuzumab, fludarabine, and melphalan RIC HCT. Blood. 2015;127(4):503–513. - PubMed
-
- Kanda J, Lopez RD, Rizzieri DA. Alemtuzumab for the prevention and treatment of graft-versus-host disease. Int J Hematol. 2011;93(5):586–593. - PubMed
-
- Kottaridis PD, Milligan DW, Chopra R, et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood. 2000;96(7):2419–2425. - PubMed
-
- Perez-Simon JA, Kottaridis PD, Martino R, et al. Nonmyeloablative transplantation with or without alemtuzumab: Comparison between 2 prospective studies in patients with lymphoproliferative disorders. Blood. 2002;100(9):3121–3127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

