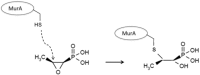New microbiological aspects of fosfomycin
- PMID: 31131587
- PMCID: PMC6555166
New microbiological aspects of fosfomycin
Abstract
The discovery of fosfomycin more than 40 years ago was an important milestone in antibiotic therapy. The antibiotic's usefulness, alone or in combination, for treating infections caused by multidrug-resistant microorganisms is clearer than ever. Both the European Medicines Agency and the US Food and Drug Administration have open processes for reviewing the accumulated information on the use of fosfomycin and the information from new clinical trials on this compound. The agencies' objectives are to establish common usage criteria for Europe and authorize the sale of fosfomycin in the US, respectively. Fosfomycin's single mechanism of action results in no cross-resistance with other antibiotics. However, various fosfomycin-resistance mechanisms have been described, the most important of which, from the epidemiological standpoint, is enzymatic inactivation, which is essentially associated with a gene carrying a fosA3-harboring plasmid. Fosfomycin has been found more frequently in Asia in extended-spectrum beta-lactamase-producing and carbapenemase-producing Enterobacterales. Although fosfomycin presents lower intrinsic activity against Pseudomonas aeruginosa compared with that presented against Escherichia coli, fosfomycin's activity has been demonstrated in biofilms, especially in combination with aminoglycosides. The current positioning of fosfomycin in the therapeutic arsenal for the treatment of infections caused by multidrug-resistant microorganisms requires new efforts to deepen our understanding of this compound, including those related to the laboratory methods employed in the antimicrobial susceptibility testing study.
Conflict of interest statement
RC has participated in training activities organized by ERN, Pfizer and MDS.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical