Schedule-dependent potentiation of chemotherapy drugs by the hypoxia-activated prodrug SN30000
- PMID: 31131698
- PMCID: PMC6741573
- DOI: 10.1080/15384047.2019.1617570
Schedule-dependent potentiation of chemotherapy drugs by the hypoxia-activated prodrug SN30000
Abstract
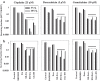
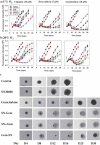
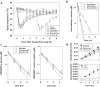
Hypoxia-activated prodrugs (HAPs) are hypothesized to improve the therapeutic index of chemotherapy drugs that are ineffective against tumor cells in hypoxic microenvironments. SN30000 (CEN-209) is a benzotriazine di-N-oxide HAP that potentiates radiotherapy in preclinical models, but its combination with chemotherapy has not been explored. Here we apply multiple models (monolayers, multicellular spheroids and tumor xenografts) to identify promising SN30000/chemotherapy combinations (with chemotherapy drugs before, during or after SN30000 exposure). SN30000, unlike doxorubicin, cisplatin, gemcitabine or paclitaxel, was more active against cells in spheroids than monolayers by clonogenic assay. Combinations of SN30000 and chemotherapy drugs in HCT116/GFP and SiHa spheroids demonstrated hypoxia-and schedule-dependent potentiation of gemcitabine or doxorubicin in growth inhibition and clonogenic assays. Co-administration with SN30000 suppressed clearance of gemcitabine in NIH-III mice, likely due to SN30000-induced hypothermia which also modulated extravascular transport of gemcitabine in tumor tissue as assessed from its diffusion through HCT116 multicellular layer cultures. Despite these systemic effects, the same schedules that gave therapeutic synergy in spheroids (SN30000 3 h before or during gemcitabine, but not gemcitabine 3 h before SN30000) enhanced growth delay of HCT116 xenografts without increasing host toxicity. Identification of hypoxic and S-phase cells by immunohistochemistry and flow cytometry established that hypoxic cells initially spared by gemcitabine subsequently reoxygenate and re-enter the cell cycle, and that this repopulation is prevented by SN30000 only when administered with or before gemcitabine. This illustrates the value of spheroids in modeling tumor microenvironment-dependent drug interactions, and the potential of HAPs for overcoming hypoxia-mediated drug resistance.
Keywords: Hypoxia-activated prodrugs; SN30000 (CEN-209); gemcitabine; multicellular spheroids; tumor hypoxia.
Figures



Similar articles
-
An agent-based model for drug-radiation interactions in the tumour microenvironment: Hypoxia-activated prodrug SN30000 in multicellular tumour spheroids.PLoS Comput Biol. 2018 Oct 24;14(10):e1006469. doi: 10.1371/journal.pcbi.1006469. eCollection 2018 Oct. PLoS Comput Biol. 2018. PMID: 30356233 Free PMC article.
-
Photodegradation of the benzotriazine 1,4-Di-N-oxide hypoxia-activated prodrug SN30000 in aqueous solution.J Pharm Sci. 2014 Nov;103(11):3464-3472. doi: 10.1002/jps.24099. Epub 2014 Sep 11. J Pharm Sci. 2014. PMID: 25212501
-
Identification of one-electron reductases that activate both the hypoxia prodrug SN30000 and diagnostic probe EF5.Biochem Pharmacol. 2014 Oct 15;91(4):436-46. doi: 10.1016/j.bcp.2014.08.003. Epub 2014 Aug 15. Biochem Pharmacol. 2014. PMID: 25130546
-
Design of optimized hypoxia-activated prodrugs using pharmacokinetic/pharmacodynamic modeling.Front Oncol. 2013 Dec 27;3:314. doi: 10.3389/fonc.2013.00314. Front Oncol. 2013. PMID: 24409417 Free PMC article. Review.
-
Targeting the hypoxic fraction of tumours using hypoxia-activated prodrugs.Cancer Chemother Pharmacol. 2016 Mar;77(3):441-57. doi: 10.1007/s00280-015-2920-7. Epub 2016 Jan 25. Cancer Chemother Pharmacol. 2016. PMID: 26811177 Free PMC article. Review.
Cited by
-
Patient-Derived Xenograft and Organoid Models for Precision Medicine Targeting of the Tumour Microenvironment in Head and Neck Cancer.Cancers (Basel). 2020 Dec 12;12(12):3743. doi: 10.3390/cancers12123743. Cancers (Basel). 2020. PMID: 33322840 Free PMC article. Review.
-
Clinical relevance and therapeutic predictive ability of hypoxia biomarkers in head and neck cancer tumour models.Mol Oncol. 2024 Aug;18(8):1885-1903. doi: 10.1002/1878-0261.13620. Epub 2024 Mar 1. Mol Oncol. 2024. PMID: 38426642 Free PMC article.
-
Interfering with Tumor Hypoxia for Radiotherapy Optimization.J Exp Clin Cancer Res. 2021 Jun 21;40(1):197. doi: 10.1186/s13046-021-02000-x. J Exp Clin Cancer Res. 2021. PMID: 34154610 Free PMC article. Review.
-
Targeting Hypoxia: Hypoxia-Activated Prodrugs in Cancer Therapy.Front Oncol. 2021 Jul 29;11:700407. doi: 10.3389/fonc.2021.700407. eCollection 2021. Front Oncol. 2021. PMID: 34395270 Free PMC article.
-
Medicinal Chemistry of Drugs with N-Oxide Functionalities.J Med Chem. 2024 Apr 11;67(7):5168-5184. doi: 10.1021/acs.jmedchem.4c00254. Epub 2024 Mar 29. J Med Chem. 2024. PMID: 38549449 Free PMC article. Review.
References
-
- Jain RK. Haemodynamic and transport barriers to the treatment of solid tumours. Int J Radiat Biol. 1991;60:85–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
