Hypothalamic Reproductive Endocrine Pulse Generator Activity Independent of Neurokinin B and Dynorphin Signaling
- PMID: 31132118
- PMCID: PMC6736049
- DOI: 10.1210/jc.2019-00146
Hypothalamic Reproductive Endocrine Pulse Generator Activity Independent of Neurokinin B and Dynorphin Signaling
Abstract
Context: Kisspeptin-neurokinin B (NKB)-dynorphin neurons are critical regulators of the hypothalamic-pituitary-gonadal axis. NKB and dynorphin are hypothesized to influence the frequency of GnRH pulses, whereas kisspeptin is hypothesized to be a generator of the GnRH pulse. How these neuropeptides interact remains unclear.
Objective: To probe the role of NKB in GnRH pulse generation and to determine the interactions between NKB, kisspeptin, and dynorphin in humans and mice with a complete absence of NKB.
Design: Case/control.
Setting: Academic medical center.
Participants: Members of a consanguineous family bearing biallelic loss-of-function mutations in the gene encoding NKB and NKB-deficient mice.
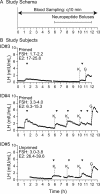
Interventions: Frequent blood sampling to characterize neuroendocrine profile and administration of kisspeptin, GnRH, and naloxone, a nonspecific opioid receptor antagonist used to block dynorphin.
Main outcome measures: LH pulse characteristics.
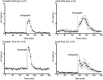
Results: Humans lacking NKB demonstrate slow LH pulse frequency, which can be increased by opioid antagonism. Mice lacking NKB also demonstrate impaired LH secretion, which can be augmented with an identical pharmacologic manipulation. Both mice and humans with NKB deficiency respond to exogenous kisspeptin.
Conclusion: The preservation of LH pulses in the absence of NKB and dynorphin signaling suggests that both peptides are dispensable for GnRH pulse generation and kisspeptin responsiveness. However, NKB and dynorphin appear to have opposing roles in the modulation of GnRH pulse frequency.
Trial registration: ClinicalTrials.gov NCT00914823 NCT01952782 NCT00494169.
Copyright © 2019 Endocrine Society.
Figures




References
-
- Schally AV, Arimura A, Kastin AJ, Matsuo H, Baba Y, Redding TW, Nair RM, Debeljuk L, White WF. Gonadotropin-releasing hormone: one polypeptide regulates secretion of luteinizing and follicle-stimulating hormones. Science. 1971;173(4001):1036–1038. - PubMed
-
- Spratt DI, Carr DB, Merriam GR, Scully RE, Rao PN, Crowley WF Jr. The spectrum of abnormal patterns of gonadotropin-releasing hormone secretion in men with idiopathic hypogonadotropic hypogonadism: clinical and laboratory correlations. J Clin Endocrinol Metab. 1987;64(2):283–291. - PubMed
-
- Chan YM, Broder-Fingert S, Paraschos S, Lapatto R, Au M, Hughes V, Bianco SD, Min L, Plummer L, Cerrato F, De Guillebon A, Wu IH, Wahab F, Dwyer A, Kirsch S, Quinton R, Cheetham T, Ozata M, Ten S, Chanoine JP, Pitteloud N, Martin KA, Schiffmann R, Van der Kamp HJ, Nader S, Hall JE, Kaiser UB, Seminara SB. GnRH-deficient phenotypes in humans and mice with heterozygous variants in KISS1/Kiss1. J Clin Endocrinol Metab. 2011;96(11):E1771–E1781. - PMC - PubMed

