Tramadol for osteoarthritis
- PMID: 31132298
- PMCID: PMC6536297
- DOI: 10.1002/14651858.CD005522.pub3
Tramadol for osteoarthritis
Abstract
Background: Tramadol is often prescribed to treat pain and is associated physical disability in osteoarthritis (OA). Due to the pharmacologic mechanism of tramadol, it may lead to fewer associated adverse effects (i.e. gastrointestinal bleeding or renal problems) compared to non-steroidal anti-inflammatory drugs (NSAIDs). This is an update of a Cochrane Review originally published in 2006.
Objectives: To determine the benefits and harms of oral tramadol or tramadol combined with acetaminophen or NSAIDs in people with osteoarthritis.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and Embase databases, as well as the US National Institutes of Health and World Health Organization trial registries up to February 2018. We searched the LILACS database up to August 2015.
Selection criteria: We included randomized controlled trials (RCTs) that evaluated the effect of tramadol, or tramadol in combination with acetaminophen (paracetamol) or NSAIDs versus placebo or any comparator in people with osteoarthritis.
Data collection and analysis: We used standard methodologic procedures expected by Cochrane.
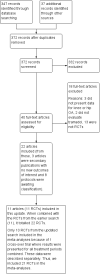
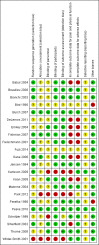
Main results: We included 22 RCTs (11 more than the previous review) of which 21 RCTs were included in meta-analyses for 3871 participants randomized to tramadol alone or tramadol in combination with another analgesic and 2625 participants randomized to placebo or active control. Seventeen studies evaluated tramadol alone and five evaluated tramadol plus acetaminophen. Thirteen studies used placebo controls and eleven studies used active controls (two trials had both placebo and active arms). The dose of tramadol ranged from 37.5 mg to 400 mg daily; all doses were pooled. Most trials were multicenter with a mean duration of two months. Participants were predominantly women with hip or knee osteoarthritis, with a mean age of 63 years and moderate to severe pain. There was a high risk of selection bias as only four trials reported both adequate sequence generation and allocation concealment. There was a low risk for performance bias as most studies blinded participants. There was a high risk of attrition bias as 10/22 trials showed incomplete outcome data. Most of the trials were funded by the pharmaceutical industry.Moderate quality evidence (downgraded due to risk of bias) indicated that tramadol alone and in combination with acetaminophen had no important benefit on pain reduction compared to placebo control (tramadol alone: 4% absolute improvement, 95% confidence interval (CI) 3% to 5%; 8 studies, 3972 participants; tramadol in combination with acetaminophen: 4% absolute improvement, 95% CI 2% to 6%; 2 studies, 614 participants).Fifteen out of 100 people in the tramadol group improved by 20% (which corresponded to a clinically important difference in pain) compared to 10/100 in the placebo group (5% absolute improvement). Twelve out of 100 people improved by 20% in the tramadol in combination with acetaminophen group compared to 7/100 in the placebo group (5% absolute improvement).Moderate quality evidence (downgraded due to risk of bias) indicated that tramadol alone and in combination with acetaminophen led to no important benefit in physical function compared to placebo (tramadol alone: 4% absolute improvement, 95% CI 2% to 6%; 5 studies, 2550 participants; tramadol in combination with acetaminophen: 4% absolute improvement, 95% CI 2% to 7%; 2 studies, 614 participants).Twenty-one out of 100 people in the tramadol group improved by 20% (which corresponded to a clinically important difference in physical function) compared to 16/100 in the placebo group (5% absolute improvement). Fifteen out of 100 people improved by 20% in the tramadol in combination with acetaminophen group compared to 10/100 in the placebo group (5% absolute improvement).Moderate quality evidence (downgraded due to risk of bias) indicated that, compared to placebo, there was a greater risk of developing adverse events with tramadol alone (risk ratio (RR) 1.34, 95% CI 1.24 to 1.46; 4 studies, 2039 participants) and tramadol in combination with acetaminophen compared to placebo (RR 1.91, 95% CI 1.32 to 2.76; 1 study, 308 participants). This corresponded to a 17% increase (95% CI 12% to 23%) with tramadol alone and 22% increase (95% CI 8% to 41%) with tramadol in combination with acetaminophen.The three most frequent adverse events were nausea, dizziness and tiredness. Moderate quality evidence (downgraded due to risk of bias) indicated that there was a greater risk of withdrawing from the study because of adverse events with tramadol alone compared to placebo (RR 2.64, 95% CI 2.17 to 3.20; 9 studies, 4533 participants), which corresponded to a 12% increase (95% CI 9% to 16%).Low quality evidence (downgraded due to risk of bias and inconsistency) indicated that there was a greater risk of withdrawing from the study because of adverse events with tramadol in combination with acetaminophen compared to placebo (RR 2.78, 95% CI 1.50 to 5.16; 2 studies, 614 participants), which corresponded to a 8% absolute improvement (95% CI 2% to 19%).Low quality evidence (downgraded due to risk of bias and imprecision) indicated that there was a greater risk of developing serious adverse events with tramadol alone compared to placebo (110/2459 participants with tramadol compared to 22/1153 participants with placebo; RR 1.78, 95% CI 1.11 to 2.84; 7 studies, 3612 participants), which corresponded to a 1% increase (95% CI 0% to 4%). There were no serious adverse events reported in one small study (15 participants) of tramadol with acetaminophen compared to placebo.
Authors' conclusions: Moderate quality evidence indicates that compared to placebo, tramadol alone or in combination with acetaminophen probably has no important benefit on mean pain or function in people with osteoarthritis, although slightly more people in the tramadol group report an important improvement (defined as 20% or more). Moderate quality evidence shows that adverse events probably cause substantially more participants to stop taking tramadol. The increase in serious adverse events with tramadol is less certain, due to the small number of events.
Conflict of interest statement
KTA: none.
JB: none.
VW: none.
LM: none.
PJ: Peter Jüni has received research grants from Astra Zeneca, Biotronik, Biosensors International, Eli Lilly and The Medicines Company, that has been paid to the institution. Peter serves as unpaid member of the steering group of trials funded by Astra Zeneca, Biotronik, Biosensors, St. Jude Medical and The Medicines Company.
AWSR: none.
EH: none.
JV: none.
TEH: none.
GW: none.
PT: Travel and accommodation for OMERACT meetings ‐ a registered non‐profit independent medical research organization, OMERACT, whose goal is to improve and advance the health outcomes for patients suffering from musculoskeletal conditions. OMERACT receives unrestriced educational grants from the American College of Rheumatology, European League of Rheumatology and several pharmaceutical companies listed below which is used to support fellows, international patient groups and support a major international bi‐annual conference which results in many peer reviewed publications; Amgen, Astra Zeneca, Bristol Myers Squibb, Celgene, EliLilly, Genentech/Roche, Genzyme/Sanofi, Horizon Pharma Inc, Merck, Novartis, Pfizer, PPD, Quintiles, Regeneron, Savient, Takeda Pharmaceutical, UCB Group, Vertex, Forest, Bioiberica Independent Committee Member for clinical trial Data Safety Monitoring Boards for FDA approved trials being conducted by UCB Biopharma GmbH & SPRL, Parexel International, and Prahealth Sciences. Independent medical consultation professional services for CHEOR Solutions (Canada) Ltd., Innovative Science Solutions LLC. An advisory committee member of the Canadian Reformulary Group Inc., a company that reviews the evidence for health insurance companies employer drug plans.
Figures

Update of
-
Tramadol for osteoarthritis.Cochrane Database Syst Rev. 2006 Jul 19;(3):CD005522. doi: 10.1002/14651858.CD005522.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2019 May 27;5:CD005522. doi: 10.1002/14651858.CD005522.pub3. PMID: 16856101 Updated.
References
References to studies included in this review
Babul 2004 {published data only}
-
- Babul N, Noveck R, Chipman H, Roth SH, Gana T, Albert K. Efficacy and safety of extended‐release, once‐daily tramadol in chronic pain: a randomized 12‐week clinical trial in osteoarthritis of the knee. Journal of Pain Symptom Management 2004;28(1):59‐71. - PubMed
Beaulieu 2008 {published data only}
Bianchi 2003 {published data only}
-
- Bianchi M, Broggini M, Balzarini P, Baratelli E, Ferrario P, Panerai AE, et al. Effects of tramadol on synovial fluid concentrations of substance P and interleukin‐6 in patients with knee osteoarthritis: comparison with paracetamol. International Immunopharmacology 2003;3(13‐14):1901‐8. - PubMed
Bird 1995 {published data only}
-
- Bird HA, Hill J, Stratford ME, Fenn GC, Wright V, Fleischmann RM, et al. A double‐blind cross‐over study comparing the analgesic efficacy of tramadol with pentazocine in patients with osteoarthritis. Journal of Drug Development & Clinical Practice 1995;7(7):181‐8. [MEDLINE: ]
Burch 2007 {published data only}
-
- Burch F, Fishman R, Messina N, Corser B, Radulescu F, Sarbu A, et al. A comparison of the analgesic efficacy of tramadol Contramid OAD versus placebo in patients with pain due to osteoarthritis. Journal of Pain and Symptom Management 2007;34(3):328‐38. - PubMed
DeLemos 2011 {published data only}
-
- DeLemos BP, Xiang J, Benson C, Gana TJ, Pascual ML, Rosanna R, et al. Tramadol hydrochloride extended‐release once‐daily in the treatment of osteoarthritis of the knee and/or hip: a double blind, randomized, dose‐ranging Trial. American Journal of Therapeutics 2011;18:216‐26. - PubMed
Emkey 2004 {published data only}
-
- Emkey R, Rosenthal N, Wu SC, Jordan D, Kamin M. Efficacy and safety of tramadol/acetaminophen tablets (Ultracet) as add‐on therapy for osteoarthritis pain in subjects receiving a COX‐2 nonsteroidal antiinflammatory drug: a multicenter, randomized, double‐blind, placebo‐controlled trial. Journal of Rheumatology 2004;31(1):150‐6. - PubMed
Fishman 2007 {published data only}
-
- Fishman R, Kistler CJ, Ellerbusch MT, Aparicio RT, Swami SS, Shirley ME, et al. Efficacy and safety of 12 weeks of osteoarthritic pain therapy with once‐daily tramadol (tramadol Contramid(R) OAD). Journal of Opioid Management 2007;5:273‐80. - PubMed
Fleischmann 2001 {published data only}
-
- Fleischmann RM, Caldwell JR, Roth SH, Tesser JR, Olson W, Kamin M. Tramadol for the treatment of joint pain associated with osteoarthritis: a randomized, double‐blind, placebo‐controlled trial. Current Therapeutic Research, Clinical & Experimental 2001;62(2):113‐28. [MEDLINE: ]
Fujii 2014 {published data only}
Gana 2006 {published data only}
-
- Florete OG, Xiang J, Vorsanger GJ. Effects of extended‐release tramadol on pain‐related sleep parameters in patients with osteoarthritis. Expert Opinion Pharmacotherapy 2008;9(11):1817‐27. - PubMed
-
- Gana TJ, Pascual ML, Fleming RR, Schein JR, Janagap CC, Xiang J, Vorsanger GJ, 023 Study Group. Extended‐release tramadol in the treatment of osteoarthritis: a multicenter, randomized, double‐blind, placebo‐controlled clinical trial. Curr Med Res Opin 2006;22(7):1391‐1401. - PubMed
-
- Kosinski M, Janagap C, Gajria K, Schein J, Freedman J. Pain relief and pain‐related sleep disturbance with extended‐release tramadol in patients with osteoarthritis. Current Medical Research and Opinion 2007;23(7):1615‐26. - PubMed
-
- Vorsanger G, Xiang J, Jordan D, Farrell J. Post hoc analysis of a randomized, double‐blind, placebo‐controlled efficacy and tolerability study of tramadol extended release for the treatment of osteoarthritis pain in geriatric patients. Clinical Therapeutics 2007;29(Suppl):2520‐35. - PubMed
Jensen 1994 {published data only}
-
- Jensen EM, Ginsberg F. Tramadol versus dextropropoxyphene in the treatment of osteoarthritis: a short term double‐blind study. Drug Investigation 1994;8(4):211‐8.
Karlsson 2009 {published data only}
-
- Karlsson M, Berggren A. Efficacy and safety of low‐dose transdermal buprenorphine patches (5, 10, and 20 pg/h) versus prolonged‐release tramadol tablets (75, 100, 150, and 200 mg) in patients with chronic osteoarthritis pain: a 12‐Week, randomized, open label, controlled, parallel‐group noninferiority study. Clinical Therapeutics 2009;31(3):503‐13. - PubMed
Kean 2009 {published data only}
-
- Kean WF, Bouchard S, Gossen ER. Women with pain due to osteoarthritis: the efficacy and safety of a once‐daily formulation of tramadol. American Academy of Pain Medicine 2009;10(6):1001‐11. - PubMed
Malonne 2004 {published data only}
-
- Malonne H, Coffiner M, Sonet B, Sereno A, Vanderbist F. Efficacy and tolerability of sustained‐release tramadol in the treatment of symptomatic osteoarthritis of the hip or knee: a multicenter, randomized, double‐blind, placebo‐controlled study. Clinical Therapeutics 2004;26(11):1774‐82. - PubMed
Park 2012 {published data only}
-
- Park K‐S, Choi J‐J, Kim W‐U, Min J‐K, Park S‐H, Cho C‐S. The efficacy of tramadol/acetaminophen combination tablets (Ultracet©) as add‐on and maintenance therapy in knee osteoarthritis pain inadequately controlled by nonsteroidal anti‐inflammatory drug (NSAID). Clinical Rheumatology 2012;31:317‐23. - PubMed
Pavelka 1998 {published data only}
-
- Pavelka K, Peliskova Z, Stehlikova H, Ratcliffe S, Repas C. Intraindividual differences in pain relief and functional improvement in osteoarthritis with diclofenac or tramadol. Clinical Drug Investigation 1998;16(6):421‐9. - PubMed
Peeva 2010 {published data only}
-
- Peeva E, Beals CR, Bolognese JA, Kivitz AJ, Taber L, Harman A, et al. A walking model to assess the onset of analgesia in osteoarthritis knee pain. Osteoarthritis and Cartilage 2010;18:646‐53. - PubMed
Schnitzer 1999 {published data only}
-
- Schnitzer TJ, Kamin M, Olson WH. Tramadol allows reduction of naproxen dose among patients with naproxen‐responsive osteoarthritis pain: a randomized, double‐blind, placebo‐controlled study. Arthritis & Rheumatism 1999;42(7):1370‐7. - PubMed
Silverfield 2002 {published data only}
-
- Silverfield JC, Kamin M, Wu SC, Rosenthal N. Tramadol/acetaminophen combination tablets for the treatment of osteoarthritis flare pain: a multicenter, outpatient, randomized, double‐blind, placebo‐controlled, parallel‐group, add‐on study. Clinical Therapeutics 2002;24(2):282‐97. - PubMed
Thorne 2008 {published data only}
-
- Thorne C, Beaulieu AD, Callaghan DJ, O'Mahony WF, Bartlett JM, Knight R, et al. A randomized, double‐blind, crossover comparison of the efficacy and safety of oral controlled‐release tramadol and placebo in patients with painful osteoarthritis. Pain Research & Management 2008;13(2):93‐102. - PMC - PubMed
Wilder‐Smith 2001 {published data only}
-
- Wilder‐Smith CH, Hill L, Spargo K, Kalla A. Treatment of severe pain from osteoarthritis with slow‐release tramadol or dihydrocodeine in combination with NSAID's: a randomised study comparing analgesia, antinociception and gastrointestinal effects. Pain 2001;91(1‐2):23‐31. - PubMed
References to studies excluded from this review
Adams 2006 {published data only}
-
- Adams EH, Breiner S, Cicero TJ, Geller A, Inciardi JA, Schnoll SH, et al. A comparison of the abuse liability of tramadol, NSAIDs, and hydrocodone in patients with chronic pain. Journal of Pain and Symptom Management 2006;31(5):465‐76. - PubMed
Argoff 2009 {published data only}
Avouac 2007 {published data only}
-
- Avouac J, Gossec L, Dougados M. Efficacy and safety of opioids for osteoarthritis: a meta‐analysis of randomized controlled trials. Osteoarthritis Cartilage 2007;15(8):957‐65. - PubMed
Choi 2007 {published data only}
-
- Choi CB, Song JS, Kang YM, Suh CH, Lee J, Choe JY, et al. A 2‐week, multicenter, randomized, double‐blind, double‐dummy, add‐on study of the effects of titration on tolerability of tramadol/acetaminophen combination tablet in Korean adults with knee osteoarthritis pain. Clinical Therapeutics 2007;29(7):1381‐9. - PubMed
Di Lorenzo 2010 {published data only}
-
- Lorenzo L, Foti C, Forte AM, Palmieri E, Formisano R, Vatakencherry A, et al. The addition of tramadol as a second opioid may improve pain relief in severe osteoarthritis: a prospective study. Pain Practice 2010;10(6):540‐7. - PubMed
Estrada 2006 {published data only}
-
- Estrada CdlP, Rodríguez IL, Rodríguez MR, Naranjo AM. Preemptive analgesia with tramadol and diclofenac in maxillofacial surgery [Analgesia preventiva con tramadol y diclofenaco en cirugía maxilofacial]. Revista Columbiana de Anestesiología 2006;34(1):15‐9.
Estrada 2007 {published data only}
-
- Estrada CdlP, Pérez MF. Comparative study of tramadol versus meperidine in knee surgery under regional anesthesia [Estudio comparativo entre tramadol vs meperidina en cirugía de rodilla bajo anestesia regional]. Revista Colombiana de Anestesiología 2007;35(4):273‐7.
Florete 2008 {published data only}
-
- Florete OG, Xiang J, Vorsanger GJ. Effects of extended‐release tramadol on pain‐related sleep parameters in patients with osteoarthritis. Expert Opinion on Pharmacotherapy 2008;9(11):1817‐27. - PubMed
Grupo Empresarial Químico‐Farmacéutico 2010 {published data only}
-
- Grupo Empresarial Químico‐Farmacéutico QUIMEFA. Tramadol, coated tablets [Tramadol, tabletas revestidas]. Revista Cubana de Farmacia 2010;44(2):276‐9.
Mariconti 2008 {published data only}
-
- Mariconti P, Collini R. Tramadol SR in arthrosic and neuropathic pain. Minerva Anestesiologica 2008;74(3):63‐8. - PubMed
McMahon 2008 {published data only}
-
- McMahon K, Nelson M, Jones G. Treating the symptoms of osteoarthritis – oral treatments. Australian Family Physician 2008;37(3):133‐5. - PubMed
McMeniman 2010 {published data only}
-
- McMeniman TJ, McMeniman PJ, Myers PT, Hayes DA, Cavdarski A, Wong MS, et al. Femoral nerve block vs fascia iliaca block for total knee arthroplasty postoperative pain control: a prospective, randomized controlled trial. Journal of Arthroplasty 2010;25(8):1246‐9. - PubMed
Olaya 2011 {published data only}
-
- Olaya HA, Hernández MA, Gómez UE, Valencia MH. Pharmacological management of neuropathic pain, current evidence [Manejo farmacológico del dolor neuropático, evidencia actual]. Acta Neurologica Colombiana 2011;27(Suppl 2):74‐86.
Pascual 2007 {published data only}
-
- Pascual ML, Fleming RR, Gana TJ, Vorsanger GJ. Open‐label study of the safety and effectiveness of long‐term therapy with extended‐release tramadol in the management of chronic nonmalignant pain. Current Medical Research and Opinion 2007;23(10):2531‐42. - PubMed
Rauck 2006 {published data only}
-
- Rauck RL, Ruoff GE, McMillen JI. Comparison of tramadol and acetaminophen with codeine for long‐term pain management in elderly patients. Current Therapeutic Research, Clinical & Experimental 2006;55(12):1‐15. [MEDLINE: ]
Stitik 2006 {published data only}
-
- Stitik TP, Altschuler E, Foye PM. Pharmacotherapy of osteoarthritis. American Journal of Physical Medicine and Rehabilitation 2006;85(11 Suppl):S15‐28. - PubMed
Turhanoğlu 2010 {published data only}
-
- Turhanoğlu AD, Güler H, İnanoğlu K, Turhanoğlu S. Tramadol iontophoresis added to treatment of knee osteoarthritis. Archives of Rheumatology 2010;25(4):174‐8.
Vorsanger 2007 {published data only}
-
- Vorsanger G, Xiang J, Jordan D, Farrell J. Post hoc analysis of a randomized, double‐blind, placebo‐controlled efficacy and tolerability study of tramadol extended release for the treatment of osteoarthritis pain in geriatric patients. Clinical Therapeutics 2007;29(Suppl):2520‐35. - PubMed
References to studies awaiting assessment
Additional references
ACR 2000
-
- American College of Rheumatology (ACR) Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee. Arthritis & Rheumatology 2000;43:1905‐15. - PubMed
Altman 1996
-
- Altman R, Brandt K, Hochberg M, Moskowitz R, Bellamy N, Bloch DA, et al. Design and conduct of clinical trials in patients with osteoarthritis: recommendations from a task force of the Osteoarthritis Research Society. Results from a workshop. Osteoarthritis and Cartilage 1996;4(4):217‐43. - PubMed
Bellamy 1997
-
- Bellamy N, Kirwan J, Boers M, Brooks P, Strand V, Tugwell P, et al. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip, and hand osteoarthritis. consensus development at OMERACT III. Journal of Rheumatology 1997;24(4):799‐802. - PubMed
Bjordal 2004
Buckwalter 2004
-
- Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research [Review]. Clinical Orthopaedics & Related Research 2004;427 Suppl:S6‐S15. - PubMed
Cross 2014
-
- Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Annals of the Rheumatic Diseases 2014;73:1323‐30. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Dieppe 2005
-
- Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet 2005;365(9463):965‐73. - PubMed
Egger 2001
-
- Egger M, Smith GD. Principles of and procedures for systematic reviews. Systematic reviews in health care: meta‐analysis in context. BMJ Publishing Group, 2001:23‐42.
Fortin 2006
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Green 2003
-
- Green CR, Anderson KO, Baker TA, Campbell LC, Decker S, Fillingim RB, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Medicine 2003;4(3):277‐94. - PubMed
Gøtzsche 2007
-
- Gøtzsche PC, Hróbjartsson A, Maric K, Tendal B. Data extraction errors in meta‐analyses that use standardized mean differences. JAMA 2007;298(4):430–7. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hochberg 2012
-
- Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care & Research 2012;64(4):465‐74. - PubMed
Jett 1997
-
- Jett MF, McGuirk J, Waligora D, Hunter JC. The effects of mexiletine, desipramine and fluoxetine in rat models involving central sensitization. Pain 1997;69(1‐2):161‐9. [MEDLINE: ] - PubMed
Jevsevar 2013
-
- Jevsevar DS. Treatment of Osteoarthritis of the Knee: Evidence‐Based Guideline, 2nd Edition. Journal of the American Academy of Orthopaedic Surgeons 2013;21:571‐6. - PubMed
Jüni 2006
-
- Jüni P, Reichenbach S, Dieppe P. Osteoarthritis: rational approach to treating the individual. Best Practice & Research. Clinical Rheumatology 2006;20(4):721‐40. - PubMed
Kalso 2004
-
- Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non‐cancer pain: systematic review of efficacy and safety. Pain 2004;112(3):372‐80. - PubMed
Kean 2004
-
- Kean WF, Kean R, Buchanan WW. Osteoarthritis: symptoms, signs and source of pain. Inflammopharmacology 2004;12(1):3‐31. - PubMed
Lammert 2014
-
- Lammert E, Zeeb M. Metabolism of Human Diseases: Organ Physiology and Pathophysiology. Vienna: Springer, 2014.
Lawrence 2008
Lesnoff‐Caravaglia 2007
-
- Lesnoff‐Caravaglia G. Health Aspects of Aging: the Experience of Growing Old. Springfield (IL): Charles C Thomas Publisher, 2007.
Murray 2012
-
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197‐223. - PubMed
NCCCC 2008
-
- National Collaborating Centre for Chronic Conditions. Osteoarthritis National Clinical Guideline for Care and Management in Adults. London: Royal College of Physicians, 2008. - PubMed
Nissen 2016
-
- Nissen SE, Yeomans ND, Solomon DH, Lüscher TF, Libby P, Husni, ME, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. New England Journal of Medicine 2016;375:2519‐29. - PubMed
Norman 2001
-
- Norman GR, Sridhar FG, Guyatt GH, Walter SD. Relation of distribution‐and anchor‐based approaches in interpretation of changes in health‐related quality of life. Medical Care 2001;39(10):1039‐47. - PubMed
Pelletier 2016
-
- Pelletier JP, Martel‐Pelletier J, Rannou F, Cooper C. Efficacy and safety of oral NSAIDs and analgesics in the management of osteoarthritis: evidence from real‐life setting trials and surveys. Seminars in Arthritis and Rheumatism 2016;45:S22‐7. - PubMed
Pendleton 2000
-
- Pendleton A, Arden N, Dougados M, Doherty M, Bannwarth B, Bijlsma JW, et al. EULAR recommendations for the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Annals of the Rheumatic Diseases 2000;59:936‐44. - PMC - PubMed
Pham 2003
-
- Pham T, Heijde D, Lassere M, Altman RD, Anderson JJ, Bellamy N, et al. Outcome variables for osteoarthritis clinical trials: the OMERACT‐OARSI set of responder criteria. Journal of Rheumatology 2003;30(7):1648‐54. - PubMed
Reginster 2002
-
- Reginster JY. The prevalence and burden of arthritis. Rheumatology (Oxford) 2002;41 Supp 1:3‐6. - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richmond 2010
-
- Richmond J, Hunter D, Irrgang J, Jones MH, Snyder‐Mackler L, Durme D, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of osteoarthritis (OA) of the knee. Journal of Bone and Joint Surgery 2010;92(4):990‐3. - PubMed
Riley 2002
-
- Riley JL III, Wade JB, Myers CD, Sheffield D, Papas RK, Price DD. Racial/ethnic differences in the experience of chronic pain. Pain 2002;100(3):291‐8. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Tannenbaum 2000
-
- Tannenbaum H, Peloso PM, Russell AS, Marlow B. An evidence‐based approach to prescribing NSAIDs in the treatment of osteoarthritis and rheumatoid arthritis: the second Canadian Consensus Conference. Canadian Journal of Clinical Pharmacology 2000;7 (Suppl A):4A‐16A. - PubMed
Towheed 2006
Tubach 2005
Zhang 2004
Zhang 2008
-
- Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis. Part II: OARSI evidence‐based, expert consensus guidelines. Osteoarthritis and Cartilage 2008;16:137‐62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

