Establishing a framework for neuropathological correlates and glymphatic system functioning in Parkinson's disease
- PMID: 31132378
- PMCID: PMC6692229
- DOI: 10.1016/j.neubiorev.2019.05.016
Establishing a framework for neuropathological correlates and glymphatic system functioning in Parkinson's disease
Abstract
Recent evidence has advanced our understanding of the function of sleep to include removal of neurotoxic protein aggregates via the glymphatic system. However, most research on the glymphatic system utilizes animal models, and the function of waste clearance processes in humans remains unclear. Understanding glymphatic function offers new insight into the development of neurodegenerative diseases that result from toxic protein inclusions, particularly those characterized by neuropathological sleep dysfunction, like Parkinson's disease (PD). In PD, we propose that glymphatic flow may be compromised due to the combined neurotoxic effects of alpha-synuclein protein aggregates and deteriorated dopaminergic neurons that are linked to altered REM sleep, circadian rhythms, and clock gene dysfunction. This review highlights the importance of understanding the functional role of glymphatic system disturbance in neurodegenerative disorders and the subsequent clinical and neuropathological effects on disease progression. Future research initiatives utilizing noninvasive brain imaging methods in human subjects with PD are warranted, as in vivo identification of functional biomarkers in glymphatic system functioning may improve clinical diagnosis and treatment of PD.
Keywords: Alpha-synuclein; Clock genes; Dopamine; Glymphatic system; Parkinson’s disease; REM-sleep behavior disorder.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declarations of Interests
None.
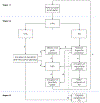
Figures

References
-
- Aarsland Bronnick K, Williams-Gray C, Weintraub D, Marder K, Kulisevsky J, Bum D, Barone P, Pagonabarraga J, Allcock L, Santangelo G, Foltynie T, Janvin C, Larsen JP, Barker RA, Emre M, 2010. Mild cognitive impairment in Parkinson disease: A multicenter pooled analysis. Neurology 75, 1062–1069. 10.1212/WNL.0b013e3181f39d0e - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

