Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine
- PMID: 31132795
- PMCID: PMC6620826
- DOI: 10.1093/brain/awz134
Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine
Abstract
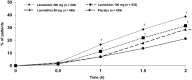
Lasmiditan, a serotonin 5-HT1F receptor agonist, was effective for acute treatment of patients with migraine in a phase 3 double-blind randomized controlled study. The current study was designed to replicate these findings in a generalizable population of patients with migraine, including those with a cardiovascular medical history. This prospective, double-blind, phase 3 multicentre study randomly assigned patients with migraine with and without aura (1:1:1:1 ratio) to oral lasmiditan 200 mg, 100 mg, 50 mg, or placebo. Patients were instructed to dose at home within 4 h of onset of migraine attack of at least moderate intensity and not improving. The primary objective was to assess the proportion of patients' headache pain-free and most bothersome symptom-free at 2 h post-dose for each dose of lasmiditan versus placebo (NCT02605174). Patients (n = 3005) were assigned and treated (n = 2583, safety population): 1938 lasmiditan (200 mg n = 528, 100 mg n = 532, and 50 mg n = 556 included in primary analysis) and 645 placebo (540 included in primary analysis). Most patients (79.2%) had ≥1 cardiovascular risk factor at baseline, in addition to migraine. Lasmiditan was associated with significantly more pain freedom at 2 h (lasmiditan 200 mg: 38.8%, odds ratio 2.3, 95% confidence interval 1.8-3.1, P < 0.001; 100 mg: 31.4%, odds ratio 1.7, 1.3-2.2, P < 0.001; 50 mg: 28.6%, odds ratio 1.5, 1.1-1.9, P = 0.003 versus placebo 21.3%) and freedom from most bothersome symptom at 2 h (lasmiditan 200 mg: 48.7%, odds ratio 1.9, 95% confidence interval 1.4-2.4, P < 0.001; 100 mg: 44.2%, odds ratio 1.6, 1.2-2.0, P < 0.001; 50 mg: 40.8%, odds ratio 1.4, 1.1-1.8, P = 0.009 versus placebo 33.5%). Treatment-emergent adverse events were reported in 253 of 649 (39.0%), 229 of 635 (36.1%), and 166 of 654 (25.4%) of patients on lasmiditan 200, 100, and 50 mg, respectively, versus 75 of 645 (11.6%) on placebo. Most adverse events were CNS-related and included dizziness, somnolence and paraesthesia. Lasmiditan was effective at 2 h post-dose for acute treatment of migraine at all oral doses tested. Efficacy and safety were consistent with the previous phase 3 study.
Keywords: efficacy; lasmiditan; migraine; phase 3; safety/tolerability.
© The Author(s) (2019). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


References
-
- Buse DC, Reed ML, Fanning KM, Kurth T, Lipton RB. Cardiovascular events, conditions, and procedures among people with episodic migraine in the US Population: results from the American Migraine Prevalence and Prevention (AMPP) study. Headache 2017; 57: 31–44. - PubMed
-
- Cameron C, Kelly S, Hsieh SC, Murphy M, Chen L, Kotb A, et al.Triptans in the acute treatment of migraine: a systematic review and network meta-analysis. Headache 2015; 55 (Suppl 4): 221–35. - PubMed
-
- Dodick DW, Lipton RB, Martin V, Papademetriou V, Rosamond W, MaassenVanDenBrink A, et al.Triptan Cardiovascular Safety Expert Panel. Consensus statement: cardiovascular safety profile of triptans (5-HT1B/1D agonists) in the acute treatment of migraine. Headache 2004; 44: 414–25. - PubMed
-
- Färkkilä M, Diener HC, Géraud G, Láinez M, Schoenen J, Harner N, et al.Efficacy and tolerability of lasmiditan, an oral 5-HT1F receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol 2012; 11: 405–13. - PubMed
-
- Ferrari MD, Färkkilä M, Reuter U, Pilgrim A, Davis C, Krauss M, et al.Acute treatment of migraine with the selective 5-HT1F receptor agonist lasmiditan—a randomised proof-of-concept trial. Cephalalgia 2010; 30: 1170–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

