Extracellular vesicles from regenerative human cardiac cells act as potent immune modulators by priming monocytes
- PMID: 31133024
- PMCID: PMC6537224
- DOI: 10.1186/s12951-019-0504-0
Extracellular vesicles from regenerative human cardiac cells act as potent immune modulators by priming monocytes
Abstract
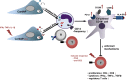
Background: Nano-sized vesicles, so called extracellular vesicles (EVs), from regenerative cardiac cells represent a promising new therapeutic approach to treat cardiovascular diseases. However, it is not yet sufficiently understood how cardiac-derived EVs facilitate their protective effects. Therefore, we investigated the immune modulating capabilities of EVs from human cardiac-derived adherent proliferating (CardAP) cells, which are a unique cell type with proven cardioprotective features.
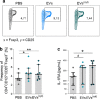
Results: Differential centrifugation was used to isolate EVs from conditioned medium of unstimulated or cytokine-stimulated (IFNγ, TNFα, IL-1β) CardAP cells. The derived EVs exhibited typical EV-enriched proteins, such as tetraspanins, and diameters mostly of exosomes (< 100 nm). The cytokine stimulation caused CardAP cells to release smaller EVs with a lower integrin ß1 surface expression, while the concentration between both CardAP-EV variants was unaffected. An exposure of either CardAP-EV variant to unstimulated human peripheral blood mononuclear cells (PBMCs) did not induce any T cell proliferation, which indicates a general low immunogenicity. In order to evaluate immune modulating properties, PBMC cultures were stimulated with either Phytohemagglutin or anti-CD3. The treatment of those PBMC cultures with either CardAP-EV variant led to a significant reduction of T cell proliferation, pro-inflammatory cytokine release (IFNγ, TNFα) and increased levels of active TGFβ. Further investigations identified CD14+ cells as major recipient cell subset of CardAP-EVs. This interaction caused a significant lower surface expression of HLA-DR, CD86, and increased expression levels of CD206 and PD-L1. Additionally, EV-primed CD14+ cells released significantly more IL-1RA. Notably, CardAP-EVs failed to modulate anti-CD3 triggered T cell proliferation and pro-inflammatory cytokine release in monocultures of purified CD3+ T cells. Subsequently, the immunosuppressive feature of CardAP-EVs was restored when anti-CD3 stimulated purified CD3+ T cells were co-cultured with EV-primed CD14+ cells. Beside attenuated T cell proliferation, those cultures also exhibited a significant increased proportion of regulatory T cells.
Conclusions: CardAP-EVs have useful characteristics that could contribute to enhanced regeneration in damaged cardiac tissue by limiting unwanted inflammatory processes. It was shown that the priming of CD14+ immune cells by CardAP-EVs towards a regulatory type is an essential step to attenuate significantly T cell proliferation and pro-inflammatory cytokine release in vitro.
Keywords: CD14+ myeloid suppressive cells; Cardiac cells; Exosomes; Extracellular vesicles; Immunomodulation; Monocytes.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







Similar articles
-
Cardiac Extracellular Vesicles (EVs) Released in the Presence or Absence of Inflammatory Cues Support Angiogenesis in Different Manners.Int J Mol Sci. 2019 Dec 17;20(24):6363. doi: 10.3390/ijms20246363. Int J Mol Sci. 2019. PMID: 31861211 Free PMC article.
-
Extracellular vesicles from human cardiovascular progenitors trigger a reparative immune response in infarcted hearts.Cardiovasc Res. 2021 Jan 1;117(1):292-307. doi: 10.1093/cvr/cvaa028. Cardiovasc Res. 2021. PMID: 32049348
-
Modulation of Immune Responses by Extracellular Vesicles From Retinal Pigment Epithelium.Invest Ophthalmol Vis Sci. 2016 Aug 1;57(10):4101-7. doi: 10.1167/iovs.15-18353. Invest Ophthalmol Vis Sci. 2016. PMID: 27537259 Free PMC article.
-
Extracellular Vesicles as Mediators of Cellular Crosstalk Between Immune System and Kidney Graft.Front Immunol. 2020 Feb 27;11:74. doi: 10.3389/fimmu.2020.00074. eCollection 2020. Front Immunol. 2020. PMID: 32180768 Free PMC article. Review.
-
Extracellular Vesicles for Immunomodulation in Tissue Regeneration.Tissue Eng Part C Methods. 2022 Aug;28(8):393-404. doi: 10.1089/ten.tec.2022.0000059. Epub 2022 Jul 19. Tissue Eng Part C Methods. 2022. PMID: 35856810 Review.
Cited by
-
Potential of mesenchymal- and cardiac progenitor cells for therapeutic targeting of B-cells and antibody responses in end-stage heart failure.PLoS One. 2019 Dec 31;14(12):e0227283. doi: 10.1371/journal.pone.0227283. eCollection 2019. PLoS One. 2019. PMID: 31891633 Free PMC article.
-
A First Phenotypic and Functional Characterization of Placental Extracellular Vesicles from Women with Multiple Sclerosis.Int J Mol Sci. 2021 Mar 12;22(6):2875. doi: 10.3390/ijms22062875. Int J Mol Sci. 2021. PMID: 33809077 Free PMC article.
-
Bioengineering Extracellular Vesicles for the Treatment of Cardiovascular Diseases.Adv Biol (Weinh). 2022 Oct;6(10):e2200087. doi: 10.1002/adbi.202200087. Epub 2022 Jul 1. Adv Biol (Weinh). 2022. PMID: 35778828 Free PMC article. Review.
-
The Systemic Effect of Ischemia Training and Its Impact on Bone Marrow-Derived Monocytes.Cells. 2024 Sep 24;13(19):1602. doi: 10.3390/cells13191602. Cells. 2024. PMID: 39404366 Free PMC article.
-
MiRNA Profiles of Extracellular Vesicles Secreted by Mesenchymal Stromal Cells-Can They Predict Potential Off-Target Effects?Biomolecules. 2020 Sep 22;10(9):1353. doi: 10.3390/biom10091353. Biomolecules. 2020. PMID: 32971982 Free PMC article.
References
-
- World Health Organization. World Health Statistics 2018: Monitoring Health for the SDGs. Sustainable development goals. 1st ed. New York: Geneva; 2018.
-
- Montanari S, Dayan V, Yannarelli G, Billia F, Viswanathan S, Connelly KA, et al. Mesenchymal stromal cells improve cardiac function and left ventricular remodeling in a heart transplantation model. J Hear Lung Transplant. 2015;34(11):1481–1488. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

