Treatment-duration is related to changes in peripheral lymphocyte counts during definitive radiotherapy for unresectable stage III NSCLC
- PMID: 31133034
- PMCID: PMC6537222
- DOI: 10.1186/s13014-019-1287-z
Treatment-duration is related to changes in peripheral lymphocyte counts during definitive radiotherapy for unresectable stage III NSCLC
Abstract
Background: To investigate the potential impact of fractionation regimes and overall treatment time (OTT) on lymphopenia during definitive radiotherapy (RT) and its associations with patient outcomes in non-small cell lung cancer (NSCLC).
Methods: Subjects consisted of 115 patients who had received definitive chemoradiation therapy (CRT) with different doses and fractions for unresectable stage III NSCLC. Clinical and laboratory records were reviewed to assess the changes in total lymphocyte counts (TLCs) during definitive RT. The associations of the TLCs with the clinical and treatment features, and outcomes were analyzed.
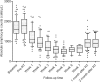
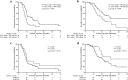
Results: The median reduction of TLCs in the entire cohort was 1300 cells/μL (interquartile range [IQR], 950-1510 cells/μL). Of all patients, 63 (54.8%) experienced severe lymphopenia (SL) (TLC nadir < 500 cells/μL), which occurred at a median of the 5th week following RT initiation, not at the completion of RT or upon treatment with maximal doses. SL risk was increased over the first 5 weeks (odds ratio [OR] = 3.455, P = 0.007), after which, no increased risk was observed (OR = 0.562, P = 0.216). The median TLCs remained low and failed to recover to the initial normal values of their pre-RT level after 2 months of RT completion. Patients without SL exhibited significantly improved progression-free survival (hazard ratio [HR] = 0.544, P = 0.010) and overall survival (HR = 0.463, P = 0.011) after controlling for confounding variables in multivariate analyses. The incidence of SL was significantly lower (71.1% reduction in risk (OR = 0.289, P = 0.007)) in patients who received hypofractionated RT with an OTT within 4 weeks, compared to those who had an OTT of more than 4 weeks (32.1% vs 62.1%, P = 0.006). Multivariate analyses revealed that OTT within 4 weeks (OR = 0.322, P = 0.032) was significantly associated with a decreased risk of developing SL after controlling for confounding factors.
Conclusions: Hypofractionated RT was significantly associated with a decreased risk of SL and improved survival during definitive radiotherapy for unresectable stage III NSCLC.
Keywords: Fractionation regimes; Non-small cell lung cancer; Overall treatment time; Total lymphocyte counts.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Ahn JS, Ahn YC, Kim JH, Lee CG, Cho EK, Lee KC, et al. Multinational randomized phase III trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent Chemoradiation in inoperable stage III non-small-cell lung Cancer: KCSG-LU05-04. J Clin Oncol. 2015;33:2660–2666. doi: 10.1200/JCO.2014.60.0130. - DOI - PubMed
-
- Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. - DOI - PMC - PubMed
-
- Osti MF, Agolli L, Valeriani M, Falco T, Bracci S, De Sanctis V, et al. Image guided hypofractionated 3-dimensional radiation therapy in patients with inoperable advanced stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;85:e157–e163. doi: 10.1016/j.ijrobp.2012.10.012. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

