Inhibition of ERK1/2 in cancer-associated pancreatic stellate cells suppresses cancer-stromal interaction and metastasis
- PMID: 31133044
- PMCID: PMC6537367
- DOI: 10.1186/s13046-019-1226-8
Inhibition of ERK1/2 in cancer-associated pancreatic stellate cells suppresses cancer-stromal interaction and metastasis
Abstract
Background: Extracellular signal-regulated kinases (ERKs) have been related to multiple cancers, including breast cancer, hepatocellular cancer, lung cancer and colorectal cancer. ERK1/2 inhibitor can suppress growth of KRAS-mutant pancreatic tumors by targeting cancer cell. However, no studies have shown the expression of ERK1/2 on pancreatic stromal and its effect on pancreatic cancer-stromal interaction.
Methods: Immunohistochemistry and western blotting were performed to detect the expression of p-ERK1/2 in pancreatic tissues and cells. Cell viability assay was used to study IC50 of ERK inhibitor on pancreatic cancer cells (PCCs) and primary cancer-associated pancreatic stellate cells (PSCs). Transwell migration, invasion, cell viability assay, senescence β-galactosidase staining were performed to determine the effect of ERK inhibitor on PCCs and PSCs in vitro and in vivo. The expression of key factors involved in autophagy and epithelial-to-mesenchymal transition (EMT) process were evaluated by western blotting. The expression of key factors related to cell invasiveness and malignancy were confirmed by qRT-PCR. Co-transplantation of PCC Organoid and PSC using a splenic xenograft mouse model was used to evaluated combined treatment of ERK inhibitor and autophagy inhibitor.
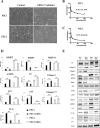
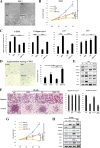
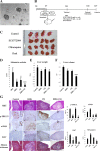
Results: Immunohistochemical staining in pancreatic tumor samples and transgenetic mice detected p-ERK1/2 expression in both cancer cells and stromal cells. In pancreatic tissues, p-ERK1/2 was strongly expressed in cancer-associated PSCs compared with cancer cells and normal PSCs. PSCs were also significantly more sensitive to ERK1/2 inhibitor treatment. Inhibition of ERK1/2 suppressed EMT transition in HMPCCs, upregulated cellular senescence markers, activated autophagy in cancer-associated PSCs; and suppressed cancer-stromal interaction, which enhanced invasiveness and viability of cancer cells. We also found that chloroquine, an autophagy inhibitor, suppressed ERK inhibition-induced autophagy and promoted PSC cellular senescence, leading to significantly decreased cell proliferation. The combination of an ERK inhibitor and autophagy inhibitor suppressed liver metastasis in a splenic pancreatic cancer organoid xenograft mouse model.
Conclusions: These data indicate that inhibition of ERK1/2 in cancer-associated pancreatic stellate cells suppresses cancer-stromal interaction and metastasis.
Keywords: Cancer–stromal interaction; Cellular senescence; ERK1/2; Pancreatic cancer; Pancreatic stellate cell.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Stewart BW, Wild CP. World cancer report 2014. World Heal Organ [Internet] 2014;1–2. Available from: https://www.who.int/cancer/publications/WRC_2014/en/.
-
- Howlader N, Noone AM, Krapcho M et al. SEER Cancer Statistics Review, 1975–2013. Natl Cancer Institute Bethesda, MD [Internet]. 2016. Available from: http://seer.cancer.gov/csr/1975_2013/.
-
- Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

