Important Trends in UCP3 Investigation
- PMID: 31133866
- PMCID: PMC6524716
- DOI: 10.3389/fphys.2019.00470
Important Trends in UCP3 Investigation
Abstract
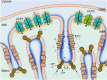
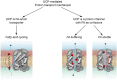
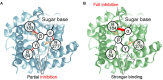
Membrane uncoupling protein 3 (UCP3), a member of the mitochondrial uncoupling protein family, was discovered in 1997. UCP3's properties, such as its high homology to other mitochondrial carriers, especially to UCP2, its short lifetime and low specificity of UCP3 antibodies, have hindered progress in understanding its biological function and transport mechanism over decades. The abundance of UCP3 is highest in murine brown adipose tissue (BAT, 15.0 pmol/mg protein), compared to heart (2.7 pmol/mg protein) and the gastrocnemius muscle (1.7 pmol/mg protein), but it is still 400-fold lower than the abundance of UCP1, a biomarker for BAT. Investigation of UCP3 reconstituted in planar bilayer membranes revealed that it transports protons only when activated by fatty acids (FA). Although purine nucleotides (PN) inhibit UCP3-mediated transport, the molecular mechanism differs from that of UCP1. It remains a conundrum that two homologous proton-transporting proteins exist within the same tissue. Recently, we proposed that UCP3 abundance directly correlates with the degree of FA β-oxidation in cell metabolism. Further development in this field implies that UCP3 may have dual function in transporting substrates, which have yet to be identified, alongside protons. Evaluation of the literature with respect to UCP3 is a complex task because (i) UCP3 features are often extrapolated from its "twin" UCP2 without additional proof, and (ii) the specificity of antibodies against UCP3 used in studies is rarely evaluated. In this review, we primarily focus on recent findings obtained for UCP3 in biological and biomimetic systems.
Keywords: cell metabolism; fatty acid beta-oxidation; mitochondria; proton transport; uncoupling protein expression pattern.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

