13C-Metabolic Flux Analysis Reveals Effect of Phenol on Central Carbon Metabolism in Escherichia coli
- PMID: 31134035
- PMCID: PMC6514248
- DOI: 10.3389/fmicb.2019.01010
13C-Metabolic Flux Analysis Reveals Effect of Phenol on Central Carbon Metabolism in Escherichia coli
Abstract
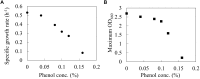
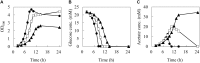
Phenol is an important chemical product that can be used in a wide variety of applications, and it is currently produced from fossil resources. Fermentation production of phenol from renewable biomass resources by microorganisms is highly desirable for sustainable development. However, phenol toxicity hampers phenol production in industrial microorganisms such as Escherichia coli. In the present study, it was revealed that culturing E. coli in the presence of phenol not only decreased growth rate, but also biomass yield. This suggests that phenol affects the carbon flow of the metabolism, but the mechanism is unknown. To investigate the effect of phenol on the flux distribution of central carbon metabolism, 13C-metabolic flux analysis (13C-MFA) was performed on cells grown under different phenol concentrations (0, 0.1, and 0.15%). 13C-MFA revealed that the TCA cycle flux reduced by 25% increased acetate production from acetyl-CoA by 30% in the presence of 0.1% phenol. This trend of flux changes was emphasized at a phenol concentration of 0.15%. Although the expression level of citrate synthase, which catalyzes the first reaction of the TCA cycle, does not change regardless of phenol concentrations, the in vitro enzyme activity assay shows that the reaction was inhibited by phenol. These results suggest that the TCA cycle flux decreased due to phenol inhibition of citrate synthase; therefore, ATP could not be sufficiently produced by respiration, and growth rate decreased. Furthermore, since carbon was lost as acetate due to overflow metabolism, the biomass yield became low in the presence of phenol.
Keywords: 13C-metabolic flux analysis; Escherichia coli; acetate overflow metabolism; citrate synthase; enzymatic assay; phenol toxicity.
Figures



References
-
- Aono R., Kobayashi H., Joblin K. N., Horikoshi K. (1994). Effects of organic solvents on growth of Escherichia coli K-12. Biosci. Biotech. Biochem. 58 2009–2014.
LinkOut - more resources
Full Text Sources

